* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Bradycardia-dependent triggered activity: relevance
Quantium Medical Cardiac Output wikipedia , lookup
Heart failure wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Jatene procedure wikipedia , lookup
Myocardial infarction wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Electrocardiography wikipedia , lookup
Atrial fibrillation wikipedia , lookup
Ventricular fibrillation wikipedia , lookup
Heart arrhythmia wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
LABORATORY INVESTIGATION
ELECTROPHYSIOLOGY
Bradycardia-dependent triggered activity: relevance
to drug-induced multiform ventricular tachycardia
JOHANNES BRACHMANN, M.D., BENJAMIN J. SCHERLAG, PH.D., LEONID V. ROSENSHTRAUKH, PH.D.,
AND RALPH LAZZARA, M.D.
Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017
ABSTRACT We used cesium chloride (CsCI) for electrophysiologic studies in canine hearts in vivo
and in vitro to examine the mechanisms underlying ventricular arrhythmias that are related to prolonged
repolarization. Cesium is known to depress normal ventricular automaticity and some experimental
arrhythmias by blocking delayed outward currents and prolonging action potential duration. In 10 dogs
in normal sinus rhythm, 1 to 1.5 mM/kg iv CsCl prolonged the QT (QU) interval and induced
ventricular ectopy in all, including multiform ventricular tachycardia. In 12 dogs with atrioventricular
block, I to 1.5 mM/kg iv CsCl produced marked suppression of idioventricular rates (from 45 + 6 to 8
4 beats/min). These low rates were then associated with bigeminy or bursts of multiform ventricular
arrhythmia. Pacing at rates of 60 beats/min or more suppressed these arrhythmias. Low doses of
tetrodotoxin (1 ,ug/kg) also abolished these bradycardia-dependent arrhythmias without affecting the
amplitude of ventricular electrograms. Tissue concentrations of cesium were determined by anatomic
absorption spectroscopy in five dogs after injection of I mM/kg CsCl. Thirty minutes after the
injection, cesium levels in Purkinje fibers were 5.3 ± 1.0 mM/kg, levels in ventricular muscle were
4.6 + 0.9 mM/kg, and levels in atrial muscle were 4.1 ± 0.8 mM/kg. In eight isolated endocardial
preparations from canine ventricles, standard microelectrode techniques were used to study the effects
of superfusion with 5 mM cesium. After 30 min, we observed early afterdepolarizations interrupting
phase 3 of Purkinje fiber action potentials that already showed prolonged repolarization.' Slowing the
rate generated single or multiple action potentials arising from partially repolarized levels of membrane
potentials ( 80 to - 65 mV). Pacing rates of 30 to 60 beats/min diminished the afterdepolarizations
and suppressed the spontaneous beats. Tetrodotoxin at a concentration of 10- g/ml, which did not
affect upstroke velocity, abolished'the afterpotentials. We conclude that cesium induced bradycardiadependent ventricular arrhythmias caused by early afterdepolarizations. These data suggest that an
inward current, probably carried by sodium ions, appears to be essential for the occurrence of this
phenomenon. The association of delayed repolarization, afterdepolarizations, and triggered activity
has similarities to the phenomenon of drug-induced prolongation of the QTU interval associated with
multiform ventricular tachycardia in humans, i.e. "torsades de pointes."
Circulation 68, No. 4, 846-856, 1983.
IN THE PAST DECADE, information has been accumulating on the occurrence of afterdepolarizations and
triggered activity in cardiac tissue and their possible
role in the generation of cardiac arrhythmias under
various experimental conditions. A substantial amount
of data concerning delayed afterdepolarizations has
been acquired from experiments with preparations in
From the Veterans Administration Medical Center and Division of
Cardiology, University of Oklahoma Health Sciences Center, Oklahoma City.
Supported by a grant from the Veterans Administration.
Address for correspondence: R. Lazzara, M.D., Oklahoma University Health Sciences Center, P.O. Box 26901, Room 3E 204, Oklahoma
City, OK 73190.
Received July 12, 1982; revision accepted June 9, 1983.
Dr. Brachmann is the recipient of research grant Br719 of the Deutsche Forschungsgemeinschaft, Federal Republic of Germany.
846
vitro that were exposed to cardiac glycosides or catecholamines. 1-9 Also, triggered activity due to delayed
afterdepolarizations has been observed in recordings
from the canine coronary sinus'0 as well as in the canine, simian, and human mitral valve muscle. 1-13 A
common denominator for these triggered arrhythmias
due to delayed afterdepolarizations has been their
tachycardia-dependent mode of induction. This electrophysiologic behavior has been used to establish
rules by which arrhythmias due to delayed afterdepolarizations in patients could be detected. 4 Observations on delayed afterdepolarizations induced by cardiac glycosides in voltage-clamp experiments provided
evidence for a transient mixed inward current due to an
increase in membrane permeability mediated by oscilCIRCULATION
LABORATORY INVESTIGATION-ELECTROPHYSIOLOGY
Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017
lation in cytosolic calcium. 15,16 This hypothesis was
substantiated by the finding that triggered activity was
suppressed by calcium-blocking drugs (e.g., verapamil) and by high concentrations of the fast channelblocker tetrodotoxin (TTX).3' 17
Because delayed afterdepolarizations generally
have shown a tachycardia dependence, they are not a
likely mechanism for bradycardia-dependent arrhythmias. The mechanisms for bradycardia-dependent arrhythmias have attracted relatively little attention. The
observation that bradycardia leads to a lengthening and
a greater variation of the relative refractory periods
throughout the heart18 19 has focused emphasis on reentrant mechanisms in the genesis of tachyarrhythmias
that are bradycardia-dependent. Bradycardia-dependent ventricular ectopy has been observed for many
years in association with complete heart block in humans20 and in other settings. Also, in recent years
considerable attention has been directed to the intriguing association of prolonged QT intervals and multiform ventricular tachycardias, called torsades de
pointes, induced by certain antiarrhythmic drugs as
well as by other factors.2' 22 It has been observed that
in these circumstances the ventricular ectopy appears
to be exacerbated by long RR intervals, i.e., bradycardia dependence. To clarify the cellular electrophysiologic mechanisms underlying the association between
delayed repolarization (prolonged QT interval), ventricular ectopy, and bradycardia dependence, we
observed the effects of cesium under conditions of
varying heart rates. Cesium is known to delay repolarization by blocking potassium currents.23 Our observations of the arrhythmogenic properties of cesium in the
experimental setting support a concept of bradycardiadependent triggered activity caused by early afterdepolarizations that are accompanied by a marked prolongation of repolarization. We hypothesize that a
similar mechanism may operate in patients with druginduced long QT intervals and associated ventricular
tachyarrhythmias.
Methods
In vivo experiments. Adult mongrel dogs weighing 10 to 20
kg were anesthetized with sodium pentobarbital (30 mg/kg, iv)
and were artificially respired with room air. A jugular vein was
cannulated for intravenous administration of drugs, and left
ventricular pressure was monitored through a polyethylene
catheter advanced into the left ventricle via the left common
carotid artery. An electrode catheter with ring electrodes 10 mm
apart was inserted into the right common carotid artery and
advanced into the noncoronary cusp of the aortic valve to record
His bundle activity.24 Vagal-induced slowing of the heart rate
was used to determine underlying idioventricular automaticity
before complete heart block was induced. Vagal-mediated
slowing was accomplished by insertion of silver wires into the
Vol. 68, No. 4, October 1983
cervical vagosympathetic trunk. Square wave pulses of 0.05
msec were delivered at 1 to 20 V and at a frequency of 20 Hz.25
A thoracotomy was performed in the right fourth intercostal
space, and the lateral surface of the right atrium and basal
portions of the right ventricle were exposed. Bipolar electrodes
consisting of pairs of stainless steel wires (0.005 inches in
diameter) were inserted into the right atrial appendage and into
the right ventricular outflow tract to provide atrial and ventricular stimulation, respectively, and to record electrograms. Pacing was achieved by delivery of electrical pulses of 2 to 10 V of
2 msec duration with an S-88 Grass stimulator and a SIU-5
isolation unit. Complete heart block was achieved by injection
of 0.3 to 0.5 ml of 37% formaldehyde into the AV node.26
Regular idioventricular rhythms obtained by vagal stimulation
or complete atrioventricular (AV) block showed no significant
difference, except for transient ventricular ectopy associated
with local damage to the ventricle on occasion. Within 2 to 5
min this intermittent activity disappeared.
Electrocardiographic lead II, His bundle electrogram, and
left ventricular pressure were continuously monitored. However, the latter was displayed intermittently on the actual recordings. All records were obtained on a multichannel oscilloscopic
photographic recorder (Electronics for Medicine, VR-12) at
paper speeds of 10 to 100 mm/sec. In addition, continuous
recordings were made in each experiment on a multichannel
magnetic tape recorder (Hewlett-Packard eight-channel) so that
sections could be replayed for analysis and for photography.
In dogs with heart block, ventricular pacing was achieved and
the responses to single and repetitive stimulation at rates from
10 to 240 beats/min were determined. When no spontaneous
ventricular rhythm was present for periods longer than 20 sec,
basic pacing at 20 beats/min was introduced. Cesium chloride
(CsCl) (0.25 to 1.0 mM/kg) was administered intravenously in
one or two doses. At a total dose of 1 to 2 mM/kg, ventricular
bigeminy or multiple ventricular ectopic beats were observed.
The response to the pacing protocol was again determined.
Thereafter, TTX was given as a 1 ,kg/kg bolus injection and the
stimulation procedure was repeated.
Tissue and plasma levels of cesium were determined in five
animals by atomic absorption spectroscopy. Control samples
were obtained from the blood and from the right atrial appendage to allow for repetitive measurements of tissue levels without
serious impairment of cardiac function. Each incision was
closed by silk ligature, and the tissue sample was resected from
an intact area of the appendage to prevent impairment of its
blood supply. Sampling was repeated at various times I to 30
min after intravenous administration of 1 mM/kg CsCl. Subsequently, the heart was removed and additional samples were
excised from free-running Purkinje strands and left ventricular
myocardium; the samples were blotted before being weighed.
We diluted 10 ml of plasma with 4.0 ml of deionized water
and vortexed it for 10 sec before analysis. Small quantities of
tissue (approximately 50 mg) were accurately weighed and 3.0
ml of deionized water was added. This material was homogenized for 75 to 90 sec, then centrifuged at 2000 g for 30 min.
The supematant was analyzed with a Varian 1200 Atomic Absorption Spectrophotometer equipped with a cesium hollow
lamp. Cesium levels were determined at a wavelength of 852.1
nm with a spectral band path of 2.0 nm. A standard curve was
established with calibrated standards ranging from 1 to 25 ,ug/
ml. The samples were analyzed, and results were obtained from
the generated standard curve. The concentrations of cesium
present were expressed as mM/l in the plasma and mM/kg in the
tissue.
In vitro experiments. Preparations were isolated from eight
mongrel dogs. After anesthesia with sodium pentobarbital (30
mg/kg, iv), a thoracotomy was performed and the hearts were
847
BRACHMANN et al.
Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017
rapidly removed and dissected at room temperature in a physiologic solution of the following composition (mM): Na+ 151.0,
K+ 4.0, Ca2+ 1.35, Mg2+ 0.5, Cl- 131.0, HCO3 24.0, H2PO4
1.8, and dextrose 5.5. The solution was equilibrated with a gas
mixture of 95% 02-5% CO2. Preparations containing Purkinje
and myocardial cells, usually including a free-running strand,
were dissected from the left ventricular endocardium. The preparation was placed into a wax-based tissue bath and superfused
with a physiologic solution at 350 to 370 C. We added CsCl in a
range of concentrations from 0.25 to 20 mM, but the observations in this report generally were made with a concentration of
5 mM, which most readily resulted in the generation of afterdepolarizations and triggered activity. The tissues were stimulated
through bipolar electrodes placed on Purkinje fiber strands.
Stimuli were rectangular pulses of 2 msec duration and variable
frequency and amplitude, delivered from pulse generators triggered by ramp generators through a stimulus isolation unit (Tektronix series 2600). Pacing protocols were similar to those in
vivo and included rates from 30 to 240 beats/min. We used
standard techniques to record intracellular and extracellular potentials. Intracellular potentials were recorded through glass
capillary microelectrodes with a tip resistance of 10 to 30
megohms and filled with 3M potassium chloride. The first stage
amplifier had high-input impedance (1014 ohms), negative capacitance feedback, and a gain of 10.
Extracellular potentials were recorded with bipolar electrodes
of fine (diameter 0.003 inches) Teflon-coated stainless steel
wires bared at the tips. The wires were led into differential
amplifiers with a gain of 200. The preamplified signals were led
into an oscilloscope with eight input channels and a dual time
base (Tektronix series 5100). The upstrokes of action potentials
were differentiated with a resistance-capacitance circuit or operational amplifier that was linear up to 1000 V/sec. Conduction
times were measured as the intervals between activation of two
close bipolar electrodes placed along a direction perpendicular
to the wavefront of excitation. The duration of action potentials
was measured to 100% repolarization. Often intracellular recordings were made from two cells widely separated in the
preparation (1 to 3 cm apart) to determine whether the changes
observed were widely distributed throughout the preparation.
Statistical analysis. Comparisons among different drugs
were performed by means of a 2-way analysis of variance.27 The
significance level for the probability (p)
were expressed as means + SD.
Results
In vivo experiments. Our preliminary reports have indicated that CsCl in doses of 0.05 to 0.25 mM/kg iv
strongly suppresses ventricular automaticity in canine
hearts in situ (figure 1).25 Figure 1, A, shows a regular
idioventricular rhythm at a rate of 42 beats/min after
introduction of complete AV nodal block. Within 2
min after CsCl, marked slowing of the spontaneous
firing in the ventricles occurred. The effect is shown in
figure 1, B, by cessation of activity after the fourth
beat. After another minute, a slow escape rhythm at a
rate of 6 beats/min resumed.
At higher concentrations of CsCl, we invariably observed the occurrence of ventricular arrhythmias. In
animals in normal sinus rhythm, the ventricular
tachyarrhythmias occurred within 30 sec of the rapid
intravenous administration of 1 to 1.5 mM/kg CsCl
and lasted for several minutes. Tachyarrhythmias were
observed in 10 animals in sinus rhythm, varying from
bigeminal premature ventricular contractions to runs
of multiform ventricular tachycardia, sometimes leading to ventricular fibrillation, which usually occurred
at doses of 1.5 mM/kg or more. A representative example is shown in figure 2. Note the sinusoidal pattern
of changing QRS and T axes resembling the clinical
arrhythmia, torsades de pointes. A slowing of the sinus
rate preceded the onset of ventricular ectopy. Since the
Control; A-V block
VR=42/min; SR=144/min
I
J i mV
L2
ttttmt
A
CsCI -40mg/kg
l
L 2-t
B
was set at .05. All
values
I
IA'.-
pl
o
.
.
-Aw
-
h~~~~fi ifIt} iI * 1
SR=144/min
secI
.
. -
.
1- 1- 1
-
-
--I.-
I
-
1 1
---
1
FIGURE 1. Effect of CsCl on nonnal ventricular automaticity in the intact heart after complete AV nodal block. A, Regular but
dissociated atrial and ventricular electrograms recorded by an electrode catheter at the AV junction (HBE) and electrocardiographic lead II (L2). The respective rates are 150 and 45 beats/min. B, After administration of 0.25 mM/kg CsCl, rapid onset of
marked depression of ventricular rhythm (VR) occurred. Note that the sinus rate (SR) remained unchanged. The HBE records
atrial activity synchronous with each p wave.
848
CIRCULATION
LABORATORY INVESTIGATION-ELECTROPHYSIOLOGY
CONTROL
CsCI 1.0 mM/kg
A
-
II
II
i
-
1
I
I
I
I
I
i
V2
CsCI 1.25 mM/kg
VA
A ANVW4
_AAAPNVWAW_
Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017
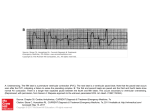
FIGURE 2. Induction of ectopic ventricular firing, multiform ventricular tachycardia, and ventricular fibrillation by intravenous
CsCl administration. In each panel are shown leads I, II, III and V2. After the administration of 1 mM/kg CsCl, the QT interval
was prolonged with the occurrence of ectopic ventricular beats interrupting the T (or U) wave and the occurrence of short bursts of
ventricular tachycardia (the end of the strip). With the administration of 1.25 mM/kg CsCl (bottom traces) there was a single
ectopic ventricular beat followed by a short run of ventricular tachycardia, followed by a longer run of ventricular tachycardia
with a sinusoidal pattem in certain leads. This latter run deteriorated into ventricular fibrillation. Note the variation in amplitude
and configuration of the TU waves in different sinus beats exemplified by the 2 sinus beats singled out by arrows.
systolic and diastolic pressure rose slightly (5% to
20%) after the administration of CsCl, the sinus slowing may have been in part a response to activation of
baroreceptors. There was always alteration of the T
wave, often the appearance of a new peak late in the T
wave or new wave following it (like a U wave), and a
prolongation of the "QU" interval measured from the
onset of the QRS to the termination of the slow waves.
When measured at a constant rate in eight animals,
the QT interval was prolonged as a QU interval by an
average of 55 + 24% (SD). At a dose of 1 mM/kg
CsCl, there was no indication of conduction delay either in change of QRS duration or in ventricular electrograms. However, at higher doses, prolongation of
the duration of the QRS complex could be observed
transiently within the first minutes when the peak serum levels of cesium were attained.
We administered 1 to 2 mM/kg CsCl intravenously
to 12 animals with heart block. Within 1 min after the
drug was dispensed we invariably noted a decline in
normal ventricular automaticity as we did with the
lower doses (figure 1). Within 3 min after injection of
CsCl, ventricular paced beats at rates less than 60
beats/min were followed by one or more ventricular
ectopic beats. The asystolic period before the inducing
ventricular beat was critically related to the ensuing
arrhythmia. A typical example is demonstrated in figure 3. In this experiment, ventricular pacing rates faster than 20 beats/min inhibited the rhythm disorder.
After cessation of pacing at various rates for 2 min, a
variable asystolic interval preceded the first escape
Vol. 68, No. 4, October 1983
ventricular complex depending on the prior paced
(overdrive) rate. In Figure 3, B, a regular bigeminy
with a coupling interval of 450 msec emerged. At
progressively longer asystolic intervals (figure 3, C
and D), typical ventricular trigeminy and quadrigeminy resulted. In figure 3, E, with the preceding
asystolic period of 8.2 sec, a short run of ventricular
tachycardia at a rate of 180 beats/min was observed;
also, note the progressively changing QRS complexes.
The rates at which the arrhythmia disappeared and
reappeared were variable among different animals and
in the same animal with time. At a dose of 1 mM/kg the
arrhythmias were transient, occurring in the first few
minutes of administration. Therefore, a steady state
did not occur and precise characterization of the relationship of the arrhythmia to heart rate was difficult.
In the animals with underlying slow ventricular rates
because of heart block, the arrhythmogenic effects
may last as long as 15 to 20 min. During this period,
repetition of pacing protocols at slow rates demonstrated tachyarrhythmias that could be suppressed by rapid
pacing. The arrhythmogenic actions of CsCl were
blunted after repetitive application. On completion of
the pacing protocol, sufficient time was available to
analyze the effects of TTX at a concentration of 1 gug/
kg, which did not diminish the amplitude of ventricular
electrograms or impair neural activation as assessed by
the unchanged rate of supraventricular automaticity
and response of atrial rate to vagal stimulation. TTX
exerted a strong depressant effect on the occurrence of
bradycardia-dependent arrhythmias within 2 min after
849
BRACHMANN et al.
I sec-]
V
Control
rU
(J
A
Cesium chloride
1mM /-kg
4.2 sec
C
i
5.2 sec
D
___
______
FIGURE 3. Effect of rate on spontaneous arrhythmias induced by CsCl (1 mM/kg) in a dog with heart
block. Electrocardiographic lead II is recorded. A,
Control idioventricular rate was 58 beats/min. After
CsCl the rate was markedly depressed and susceptible to overdrive suppression. B-E, After overdrive.,
the time interval before the first spontaneous beat is
indicated below arrows in each panel. Note that with
increasing asystolic interval the number of triggered
beats increased until in E a burst of ventricular tachycardia ensued.
6.8 sec
Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017
8.2 sec
injection. After TTX, subsequent CsCl injections
failed to initiate any more arrhythmias. In three experiments, TTX administered before the first dose of CsCl
prevented the occurrence of arrhythmias. A total dose
of 10 to 12 mM/kg CsCl was lethal in our experiments,
usually causing a marked fall in left ventricular pressure, conduction disturbances, and ventricular fibrillation.
Tissue and plasma levels of cesium in vivo. To correlate
in vivo and in vitro concentrations of cesium, we measured the tissue and plasma levels in five dogs. Tissue
levels were obtained from atrial muscle to allow for
multiple excisions of material without severely damaging the heart. There was a rapid increase in plasma
0
0
7.5-
PLASMA ATRIUM
A
t~ ~ ~PF
E 5.0
2.5-
10
20
30
min
CsCI 1mM/kg
FIGURE 4. Time course of tissue and plasma concentrations of cesium
(Cs +) after injection of 1 mM/kg CsCl. Note the rapid decline in plasma
concentrations after the early peak value compared with the slow
changes in tissue concentrations. See text for discussion. PF = Purkinje
fiber; VM = ventricular muscle.
850
concentration 1 min after injection that rapidly decreased after 5 min, whereas the peak value in the
tissue was reached after 10 min followed by a slow
decrease. In contrast, the plasma concentration continued to decline rapidly. After 30 min, the heart was
excised and additional samples from the ventricular
muscle and free-running Purkinje strands were analyzed. Both values were slightly higher than those
obtained from the atrium, but were still near the concentration chosen for superfusion of the in vitro preparation (5 mM). The plasma and tissue concentration
time-curves are shown in figure 4.
In vitro experiments. In voltage clamp experiments,
cesium ions have been shown to depress the pacemaker
current of cardiac Purkinje fibers and thereby depress
diastolic (phase 4) depolarization.23 This finding was
confirmed in our experiments. Figure 5 shows recordings from a spontaneously firing preparation. After
addition of cesium (5 mM/l), spontaneous diastolic
depolarization of the Purkinje fiber was markedly reduced and the rate was slowed from 44 beats/min to 11
beats/min. Cesium also consistently prolonged action
potential duration at a constant heart rate. The apparent
positive shift of the transition between phases 4 and 0
was not a consistent finding. In these recordings it may
reflect the transformation from latent to true pacemaker in the cell recorded.
The early effects of cesium on transmembrane potentials are illustrated by the recordings shown on figure 6 from a representative experiment. After the addition of cesium (5 mM/l) to the superfusate (figure 6,
B), no change in resting potential, action potential
amplitude, or Vmax could be discerned. However, acCIRCULATION
LABORATORY INVESTIGATION-ELECTROPHYSIOLOGY
A t t
t1
1
1
1
|
B
J 20
mV
FIGURE 5. Effect of cesium on normal Purkinje
fiber automaticity. A, before cesium, the rate of Purkinje automaticity was 46 beats/min. B, After 5 min
of 5 mM cesium, the rate decreased to 11 beats/min
associated with depression of the slope of phase 4
depolarization.
1sec
Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017
tion potential duration increased from 420 to 500
msec, while conduction velocity remained constant as
recorded at a faster sweep speed as shown in the right
section of figure 6. Note that Vmax as represented on the
second line from the top is unchanged. Within 20 min
after addition to the superfusate, TTX (10-8 g/ml) produced a shortening of action potential duration (from
500 to 380 msec), whereas Vax was only slightly diminished (figure 6, C). With cesium (5 mM/l) in the
absence of TTX, prolonged action potentials were
maintained for as long as 90 min. Some of the cellular
electrophysiologic effects observed within 15 min of
exposure to cesium and subsequent TTX are summarized in table 1. Our data indicate that cesium prolonged the action potential duration by 36% while other recorded parameters were not significantly altered.
Prolongation of the action potential (plateau) usually
preceded other alterations of the repolarization phase
in Purkinje fibers. The action potential of subendocardial myocardial fibers were also prolonged from 249
± 36 to 311 ± 47 msec (p < .01).
During continued superfusion with cesium, the rapid repolarization phase (phase 3) of the action potential
developed a terminal delay, appearing as an inflection
in the course of repolarization as illustrated by the
action potentials shown in figure 7, B, compared with
the action potentials shown in 7, A. With continued
superfusion and lengthening of the cycle length, this
delaying inflection became more prominent and assumed the form of a plateau or a positive drift (depolarization) from which action potential upstrokes arose
(figure 7, C).
The inflection delaying the course of terminal repolarization always anteceded frank depolarizations. Presumably, the recording of overt afterdepolarizations
might depend on proximity of the recording site to the
I.
I
A
I
FIGURE 6. Effect of cesium and TTX on action
potentials of canine Purkinje fibers. Top trace is the 0
potential and middle trace the upstroke velocity (dv/
dt) in all panels. A, Control. B, After 25 min of
superfusion with 5 mM cesium. Action potential duration was markedly prolonged but action potential
amplitude, resting potential, Vmax, and conduction
velocity remained unchanged. C, There was marked
shortening of the action potential duration 20 min
after addition of TTX (l0-8 g/ml). Stimulation rate,
0.5/sec. Stimuli are indicated by arrows. Vertical
calibration factors correspond to action potential and
dv/dt recordings, respectively.
B I
I
C
-14-
20mV1 5ooVIs
ioomsec
Vol. 68, No. 4, October 1983
20 1"S
851
BRACHMANN et al.
TABLE 1
Effects of cesium and TTX on action potential parameters of Purkinje fibers (mean + SD)
AP amplitude (mV) MDP (mV)
V
(V/sec)
AP duration (msec)
Control
109.4 5.1 84.1+- 3.1
Cs 5 mM
(30 min)
107.9 3.4 83.2 -+2.8
Cs 5 mM and
TTX 10- g/ml
102.4 + 6.2 80.6 ±4.1
(30 min)
465 ± 85
361±+42
439 + 82
492 74A
414 + 97
343 54B
Results were obtained from eight experiments and with a pacing cycle
length of 2000 msec.
AP = action potential; MDP - maximum diastolic potential; Cs
cesium.
Statistical comparisons (two-way analysis of variance): Ap < .05 with
respect to the control group; Bp < .05 with respect to the group superfused with Cs 5 mM alone.
Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017
site of initiation of activation in the preparation. Recordings of frank afterdepolarizations are shown in
figure 8. Note that the action potentials recorded with a
driving rate of 60 beats/min show the delaying inflection in the later third of repolarization but no spontaneous discharges (figure 8, top left), but when the preparation was permitted to fire spontaneously at a slow
rate, one of the cells showed a series of afterdepolarizations culminating in spontaneous discharge (figure
8, top right). TTX shortened the action potential, attenuated the delaying inflection at a driving rate of 60
beats/min (figure 8, bottom left), and suppressed the
afterdepolarizations and triggered firing at the slow
spontaneous rate (figure 8, bottom right). The basic
abnormality in the terminal phase of repolarization is
also seen in action potentials recorded from the other
cell, but afterdepolarizations are less apparent.
The influence of pacing on the phenomenon is illustrated in the recordings shown on figure 9. Figure 9,
top left, demonstrates a regular bigeminy with an interposed diastolic interval of 1700 msec in a spontaneously firing preparation. The membrane potential from
which the second beat originated was -73 mV, but
the maximum diastolic potential was -85 mV. An
increase in the asystolic interval to 1850 msec (figure
9, top right) was associated with a greater number of
beats linked to the escape complex. Shortening of the
cycle length to 1200 msec by regular pacing (figure 9,
lower panel) diminished the early afterdepolarizations
and the prominence of the delaying inflection, resulting in total suppression of the triggered discharges.
The early afterdepolarizations did not always result
in excitation, as demonstrated in figure 10, which
shows the recordings from a preparation that was ex852
posed to cesium for 20 min. The fiber exhibited regular bradycardia-dependent early afterdepolarizations
causing bursts of spontaneous beats. This was succeeded by an abortive action potential that was followed by decreasing oscillations of the membrane potential until the membrane potential stabilized at -66
mV. After 3.8 sec, an action potential was generated
by stimulation, resulting in repolarization to the original membrane potential (-81 mV). Thus, the membrane potential could be relatively stable at another
C O N T ROL
A.
25' CsCI 5mM
B.
1.
-
.6
I
*
C.
J"+20mV
200 Ql88C
44' CsCI 5mM
500 msec
FIGURE 7. Effect of cesium on terminal repolarization of Purkinje
fiber action potentials during pacing at 60 beats/min (A and B) and
spontaneous slow rate (C). Top trace, 0 potential; middle trace, upstroke velocity in all panels. After 25 min of superfusion with 5 mM
cesium only the action potential duration increased. Note appearance of
delaying inflections during the terminal repolarization phase (B, arrows). C, After 40 min of cesium, a spontaneous slow rate (30 beats!
min) resulted in a coupled excitation beginning during an apparent
second plateau of membrane potential late in the course of repolarization. In this preparation, the two recording sites were 2.5 cm apart near
opposite ends of the preparation.
CIRCULATION
LABORATORY INVESTIGATION-ELECTROPHYSIOLOGY
Cs
\
I ofms
J20mv
\\
l !.s
&
SE =B
l
S
==W
Cs + TTX
is
\
I OOms
~~~20mv
ls
FIGURE 8. Suppression by TTX of afterdepolarizations and triggered discharges induced by cesium.
Top left, Action potentials recorded from Purkinje
cells 1.8 cm apart driven at 60 beats/min, 55 mim
after exposure to cesium (5 mM/l). Top right, Recordings made during a slow spontaneous rate,
showing afterdepolarizations and triggered activity
more prominent in one cell than the other. Bottom
left and bottom right, Recordings taken under comparable conditions except for exposure to TTX (f08
g/ml) for 8 min.
40
Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017
more depolarized level. Further depression of the oscillatory afterpotentials occurred during the recordings
of figure 10, D. Again the resting potential stabilized at
-65 mV for 7.1 sec until pacing and associated spontaneous beats resumed. Because these oscillations
originated at a level of membrane potential more positive than the maximum diastolic potential and they
occurred before full repolarization was achieved, we
interpreted them as early afterdepolarizations.
Discussion
In the present study we used cesium to induce ventricular atrhythimias that are electrocardiographically
indistinguishable from the multiform ventricular
tachycardias sometimes induced by certain antiarrhythmic drugs and psychotropic drugs, and that are
associated with other conditions such as electrolyte
I
imbalance and marked bradycardia.21 22 The electrocardiographic-clinical entity of torsades de pointes is
characterized by markedly prolonged QT (or QU) intervals, multiform ventricular tachycardia with progressive shifts in the direction of the QRS and T vectors in a sinusoidal pattern, and exacerbation of the
ectopy at slower heart rates (bradycardia dependence).
The slow waves following the QRS often appear to
consist of both abnormal T and U waves. Usually the
TU waves are labile from beat to beat, especially with
varying cycle lengths. The phenomenon induced by
cesium in the whole animal has strikingly similar characteristics. In vitro cesium in concentrations comparable to the tissue levels measured in vivo produced
delayed repolarization and early afterdepolarizations
that generated triggered firing, especially at slower
heart rates. These cellular electrophysiologic effects
I
k
,
! I
1 sec
20
mv
II
Vol. 68, No. 4, October 1983
t
III
FIGURE 9. Effect of different rates on transmembrane potentials of Purkinje fibers recorded after 30
min administration of 5 mM cesium. Upper left, A
bigeminy was observed with a diastolic interval of
1700 msec. Each initial beat was followed regularly
by an action potential arising from an early afterdepolarization. Upper right, An increase in the diastolic cycle length to 1850 msec led to bursts of ectopic
beats each preceded by afterdepolarizations. Bottom,
After a long asystole, the first paced beat (first arrow)
induced an early afterdepolarization and associated
response. During repolarization of this beat, the second pacing impulse (second arrow) diminished the
afterdepolarization and suppressed further spontaneous activity as pacing (cycle length 1200 msec) continued.
-I
2sec
853
BRACHMANN et al.
A |
i
_0
C
-%
I
III.
~I
1I I
.Aj
t
1
s8ec
D
-
-
mosow
t
II
I
t
I
Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017
FIGURE 10. Effect of cesium on transmembrane potentials of Purkinje fibers. A and B, Bradycardia-dependent early afterdepolarization and bursts of spontaneous activity after 20 min of superfusion with 5 mM cesium. C, After 2 min, the second paced
action potential (second arrow) elicited an early afterdepolarization leading to brief oscillations of the membrane potential.
Afterward the potential remained at this depolarized level. A stimulated action potential (third arrow) introduced after 3.5 sec
caused repolarization to the former resting potential. D, After another oscillatory response, the membrane potential persisted for
6 sec at a depolarized level. Another paced beat triggered spontaneous action potentials followed by more complete repolarization.
may be the basis for the electrocardiographic changes
and arrhythmias observed in intact animals in vivo. It
should be noted that the recordings from a few cells in
the preparation may not represent all the phenomena
occurring in the intact heart. Detailed sampling of ventricular myocardial cells was not performed in these
studies. The Purkinje tissues appeared to be involved
diffusely, since virtually all sampling sites showed
similar phenomena to differing degrees.
Analysis of the tissue levels of cesium demonstrated
that these values were in the range of the concentrations in vitro. The difference in the time course of the
effects in vivo and in vitro may have been related to
rapid initial tissue uptake in vivo that may remain
relatively high intracellularly while the plasma concentrations rapidly declined. In contrast, there may
have been a continuous uptake of cesium from the
superfusate in vitro until a certain intracellular concentration was attained at which the phenomena occurred.
Cesium has been shown to depress potassium currents in Purkinje fibers as well as in other excitable
tissues 29-31 In Purkinje fibers, the inward rectifying
potassium channel is blocked by 20 mM/l cesium.23 32
Cesium in low concentrations (1 mM/l) also depressed
the pacemaker current, which has been interpreted as a
deactivating outward current, iK,23 or as an activating
inward current, is."4 To account for afterdepolarizations and excitation, it is necessary to consider inward
854
currents. Aconitine, which produces delayed repolarization, early afterdepolarizations, and anomalous
(triggered) firing35 36 (phenomena resembling those induced by cesium), enhances an inward current activated at -60 mV37 and blocked by low concentrations of
TTX.38 39 It has been shown that in normal Purkinje
fibers, a noninactivated sodium current flows during
the plateau, and this current can be blocked by concentrations of TTX lower than those required to block the
excitatory fast sodium current.40 41 In light of these
observations, we used low concentrations of TTX to
provide an indication of the implication of the sodium
current in the afterdepolarizations and anomalous excitations caused by cesium. The suppression of afterdepolarizations and abolition of triggered excitation by
low concentrations of TTX suggest that the noninactivated sodium current is involved. Cesium has not been
shown to enhance an inward current. It may be that the
"normal" noninactivated sodium current is sufficient
to result in afterdepolarizations and triggered discharges if repolarization is sufficiently delayed and
outward currents are blocked. The bradycardia
dependence of the afterdepolarizations probably relates to the prolongation of repolarization with longer
cycle lengths. Also, at shorter cycle lengths, extracellular potassium concentration increases,42 resulting in
increased potassium conductance, which would partially reverse the effects of cesium.
CIRCULATION
LABORATORY INVESTIGATION-ELECTROPHYSIOLOGY
Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017
It was not possible in this study to define precise
relationships between cycle length and early afterdepolarizations or spontaneous discharges. The relationships were variable with time and among preparations.
However, there was a consistent inverse relationship
between the occurrence of triggered firing and the
heart rate.
In experimental and clinical studies, bradycardiadependent arrhythmias have been attributed to
reentry.'9 In patients with atrial fibrillation, Langendorf et al.43 defined a specific "rule' of bigeminy"
demonstrating bradycardia-dependent increase in frequency of ectopic beats. In patients with bradycardia
due to heart block, multiform ventricular tachyarrhythmias have been observed that very closely resemble the
type described as torsades de pointes.20 44 It has been
proposed that temporal dispersion of refractoriness is
the basis for reentrant mechanisms with bradycardia. 19
However, dispersion of refractoriness cannot be a sole
mechanism for the generation de novo of ectopic beats
because it requires that an impulse interrupt the refractory period of a beat during the stable rhythm. Therefore, even under conditions of markedly dispersed refractoriness, another mechanism is required for the
initial ectopic beat(s) that might in turn result in subsequent reentrant beats. In our studies, dispersion of
refractoriness and reentry could have come into play
after one or more automatic beats. Repolarization was
abnormally prolonged and probably abnormally heterogeneous. However, dispersion of refractoriness
was not quantified. We propose that the arrhythmias
induced by cesium have as their initiating mechanism,
triggered firing due to early afterdepolarizations. It is
possible that reentry is also operative because heterogeneous delay of repolarization might produce dispersion of refractoriness. An arrhythmia resembling
torsades de pointes has been observed in ischemic dog
hearts in which mapping of activation has suggested
two competing activation sequences,45 presumably
representing two reentrant circuits.
Reports of patients treated with certain antiarrhythmic drugs and psychotropic drugs have revealed a significant incidence of tachyarrhythmias and sudden
death.2"' 22 This was related to marked prolongation of
the QT (or QU) interval, which might facilitate reentry
by inhomogeneous repolarization. Catecholamine infusion and atrial pacing to shorten the QT interval have
been useful in suppressing these arrhythmias.21 In general, the drugs that have been shown to produce these
multiform ventricular tachyarrhythmias have been
those that prolong the QT interval and delay repolarization within normal Purkinje and myocardial fibers.
Vol. 68, No. 4, October 1983
Drugs that facilitate repolarization and shorten the QT
interval, such as lidocaine, generally have not been
implicated in this phenomenon. Perhaps certain individuals are especially sensitive to the effects of certain
drugs to block outward currents and delay repolarization, and as a result these individuals are susceptible to
the generation of tachyarrhythmias. Other factors operating in certain individuals, such as myocardial hypertrophy, may also potentiate the effects of drugs that
delay repolarization. It has been shown recently that
hypertrophied myocardium in rats is prone to the development of early and delayed afterdepolarizations
under certain conditions.46 The occurrence of torsades
de pointes with hypokalemia or hypocalcemia fits with
the proposed mechanisms, since potassium conductance would be expected to be decreased in those conditions. Since torsades de pointes can occur in the
absence of drugs or electrolyte alterations under conditions of marked bradycardia, it is possible that the
delay of repolarization attendant on bradycardia alone
sometimes results in early afterdepolarizations and
automatic firing.
The hypothesis that torsades de pointes associated
with long QT intervals is based on the cellular electrophysiologic phenomena of early afterdepolarizations
and triggered firing, rests for the most part on the
similarity of the phenomena produced by cesium in
vivo in dogs to the clinical entity: the prolongation of
the QT interval, the appearance of U waves, the variability of the T and U waves, the undulating pattern of
the multiform ventricular tachycardia, and the bradycardia dependence. Further support for the hypothesis
is provided by the observation that the major metabolite of procainamide, N-acetyl procainamide, which
attains significant concentrations in humans (especially in individuals who are rapid acetylators), has been
shown to produce early afterdepolarizations associated
with markedly delayed repolarization in Purkinje fibers.4
References
1. Davis LD: Effect of changes in cycle length on diastolic depolarization produced by ouabain in canine Purkinje fibers. Circ Res 32:
206, 1973
2. Rosen MR, Gelband H, Hoffman BF: Correlation between effects
of ouabain on the canine electrogram and transmembrane potentials of isolated Purkinje fibers. Circulation 47: 65, 1973
3. Rosen MR, Danilo P Jr: Effects of tetrodotoxin, lidocaine, verapamil, and AHR-2666 on ouabain-induced delayed afterdepolarization in canine Purkinje fibers. Circ Res 46: 117, 1980
4. Ferrier GR, Saunders JH, Mendez C: A cellular mechanism for the
generation of ventricular arrhythmias by acetylstrophantidin. Circ
Res 32: 600, 1973
5. Aronson RS, Cranefield PF: Electrical activity of canine cardiac
Purkinje fibers in sodium-free, calcium-rich solutions. J Gen
Physiol 61: 786, 1973
6. Cranefield PF: The conduction of the cardiac impulse. The slow
855
BRACHMANN et al.
7.
8.
9.
10.
11.
12.
13.
14.
Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017
15.
16.
17.
18.
19.
20.
21.
22.
23.
24.
25.
26.
856
response and cardiac arrhythmias. Mount Kisco, NY, 1975, Futura
Press
Cranefield PF: Action potentials, afterpotentials and arrhythmias.
Circ Res 41: 415, 1977
Hordof AJ, Spotnitz A, Mary-Rabine L, Edie R, Rosen MR: The
cellular electropohysiologic effects of digitalis on human atrial
fibers. Circ Res 57: 223, 1978
Yeh BK, Lazzara R: Genesis of triggered and spontaneous automaticity by norepinephrine and isoproterenol in ventricular myocardium. Fed Proc 36: 1660, 1977
Wit AL, Cranefield PF: Triggered and automatic activity in the
canine coronary sinus. Circ Res 41: 435, 1977
Wit AL, Cranefield PF: Triggered activity in cardiac muscle fibers
of the simian mitral valve. Circ Res 38: 435, 1976
Wit AL, Fenoglio JJ Jr, Wagner BM, Bassett AL: Electrophysiological properties of cardiac muscle in the anterior mitral valve
leaflet in the dog. Circ Res 32: 731, 1973
Wit AL, Fenoglio JJ, Hordof AJ, Reemtsma K: Ultrastructure and
transmembrane potentials of cardiac muscle in the human anterior
mitral valve leaflet. Circulation 59: 1284, 1979
Rosen MR, Fisch C, Hoffman BF, Danilo P Jr, Lovelace DE,
Knoebel SB: Can accelerated atrioventricular junctional escape
rhythms be explained delayed afterpotentials? Am J Cardiol 45:
1272, 1980
Lederer WJ, Tsien RW: Transient inward current underlying arrhythmogenic effects of cardiotonic steroids in Purkinje fibers. J
Physiol (Lond) 263: 209, 1976
Kass RS, Tsien RW, Weingart R: Ionic basis of transient inward
current underlying arrhythmogenic effects of cardiotonic steroids
in cardiac Purkinje fibers. J Physiol (Lond) 281: 209, 1978
Mary-Rabine L, Hordof AJ, Danilo P Jr, Malm JR, Rosen ML:
Mechanisms for impulse initiation in isolated human atrial fibers.
Circ Res 47: 267, 1980
Han J, Moe GK: Nonuniform recovery of excitability in ventricular
muscle. Circ Res 24: 44, 1964
Han J, Millet D, Chizzowitz B, Moe GK: Temporal dispersion of
recovery of excitability in atrium and ventricle as a function of
heart rate. Am Heart J 71: 481, 1966
Schwartz SP: Studies on transient ventricular fibrillation. III. The
prefibrillatory mechanisms during established auriculoventricular
dissociation. Am J Med Sci 192: 153, 1936
Krikler DM, Curry PVL: Torsade de pointes, an atypical ventricular tachycardia. Brit Heart J 38: 117, 1976
Kossmann CE: Torsade de pointes: An addition to the nosography
of ventricular tachycardia. Am J Cardiol 42: 1054, 1978
Isenberg G: Cardiac Purkinje fibers: cesium as a tool to block
inward rectifying potassium currents. Pfluegers Arch 365: 99,
1976
Scherlag BJ, Abelleira JL, Samet P: Electrode catheter recording
from the His bundle and left bundle in the intact dog. In Kao FF,
Koizumi K, Vassalle M, editors: Research in physiology. Bologna,
Italy, 1971, Aulo Gaggi, p 223-238
Lazzara R, Scherlag BJ, Robinson MJ, Samet P: Selective in situ
parasympathetic control of the canine sino-atrial and atrio-ventricular nodes. Circ Res 32: 393, 1973
Scherlag BJ, Kosowsky BD, Damato AN: A technique for ventricular pacing from the His bundle of the intact heart. J Appl Physiol
22: 584, 1967
27. Barr AJ, Goodnight JH, Sall JP, Helwig JT: A user's Guide to SAS
76. Raleigh, NC, 1976, SAS Institute, pp 127-144
28. Brachmann J, Meier CF, Scherlag BJ, Kabell G, Harrison LA,
Lazzara R: Differential effects of cesium chloride on sinus node and
His-Purkinje automaticity in the normal dog heart. Circulation 62
(suppl III): 111- 173, 1980 (abst)
29. Adelmann WJ Jr, Senft JP: Voltage clamp studies on the effect of
internal cesium ion on sodium and potassium currents in the squid
giant axon. J Gen Physiol 50: 279, 1966
30. Gay LA, Stanfield PR: Cs + causes a voltage dependent block of
inward K + current in resting skeletal muscle fibers. Nature 267:
169, 1977
31. Adelmann WJ Jr, French RJ: Blocking of the squid axon potassium
channel by external cesium ions. J Physiol (Lond) 276: 13, 1978
32. Vereecke J, Isenberg G, Carmeliet E: K efflux through inward
rectifying K channels in voltage clamped Purkinje fibers. Pfluegers
Arch 384: 207, 1980
33. Noble D, Tsien RW: The kinetics and rectifier properties of the
slow potassium current in cardiac Purkinje fibers. J Physiol 195:
185, 1968
34. DiFrancesco D: A new interpretation of the pacemaker current in
calf Purkinje fibers. J Physiol 314: 359, 1981
35. Matsuda K, Hoshi T, Nameyamo S: Effects of aconitine on the
cardiac membrane potential of the dog. Jpn J Physiol 9: 419, 1981
36. Schmidt RF: Versuche mit Aconitin zum Problem der spontanen
Erregungsbildung in Herzen. Pfluegers Arch 271: 526, 1960
37. Peper K, Trautwein W: The effect of aconitine on the membrane
current in cardiac muscle. Pfluegers Arch 296: 328, 1967
38. Huang TF: Effect of tetrodotoxin and manganese ion on the aconitine induced arrhythmias of the isolated rabbit atrium and ventricle.
Jpn J Physiol 20: 435, 1970
39. Huang TF: Antiarrhythmic action of tetrodotoxin in various animal
species. Jpn J Physiol 23: 39, 1973
40. Coraboeuf E, Deroubain E, Couloumbe A: Effects of tetrodotoxin
on action potentials of the conducting system in the dog heart. Am J
Physiol 236: H561, 1979
41. Attwell D, Cohen I, Fisher D, Ohba M, Ojeda C: The steady state
TTX-sensitive ("window") sodium current in cardiac Purkinje
fibers. Pfluegers Arch 379: 137, 1979
42. Kunze D: Rate-dependent changes in extracellular potassium in the
rabbit atrium. Circ Res 41: 122, 1977
43. Langendorf R, Pick A, Winternik M: Mechanisms of intermittent
ventricular bigeminy. I. Appearance of ectopic beats dependent
upon length of the ventricular cycle, the "rule of bigeminy."
Circulation 11: 422, 1955
44. Langendorf R, Pick A: Causes and mechanisms of ventricular
asystole in advanced A-V block. In Surawicz B, Pellegrino EE,
editors: Sudden cardiac death. New York, 1964, Grune & Stratton,
pp 97-107
45. Bardy GH, Ungerleider RM, Smith WM, Ideker RE: A mechanism
of torsades de pointes in a canine model. Circulation 67: 52, 1983
46. Aronson RS: Afterpotentials and triggered activity in hypertrophied myocardium from rats with renal hypertension. Circ Res 48:
720, 1981
47. Dangman KH, Hoffman BE: Effects of N-acetylprocainamide on
cardiac Purkinje fibers. Pharmacologist 20: 150, 1978 (abst)
CIRCULATION
Bradycardia-dependent triggered activity: relevance to drug-induced multiform
ventricular tachycardia.
J Brachmann, B J Scherlag, L V Rosenshtraukh and R Lazzara
Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017
Circulation. 1983;68:846-856
doi: 10.1161/01.CIR.68.4.846
Circulation is published by the American Heart Association, 7272 Greenville Avenue, Dallas, TX 75231
Copyright © 1983 American Heart Association, Inc. All rights reserved.
Print ISSN: 0009-7322. Online ISSN: 1524-4539
The online version of this article, along with updated information and services, is located on
the World Wide Web at:
http://circ.ahajournals.org/content/68/4/846
Permissions: Requests for permissions to reproduce figures, tables, or portions of articles originally
published in Circulation can be obtained via RightsLink, a service of the Copyright Clearance Center, not the
Editorial Office. Once the online version of the published article for which permission is being requested is
located, click Request Permissions in the middle column of the Web page under Services. Further
information about this process is available in the Permissions and Rights Question and Answer document.
Reprints: Information about reprints can be found online at:
http://www.lww.com/reprints
Subscriptions: Information about subscribing to Circulation is online at:
http://circ.ahajournals.org//subscriptions/