* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Development of the Kidneys
Gene expression profiling wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Wnt signaling pathway wikipedia , lookup
Epigenetics in stem-cell differentiation wikipedia , lookup
Mir-92 microRNA precursor family wikipedia , lookup
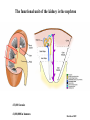
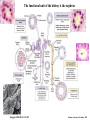
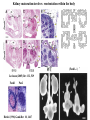
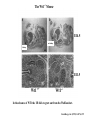
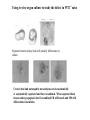
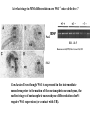
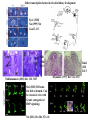
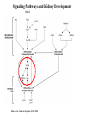
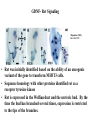
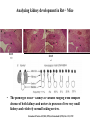
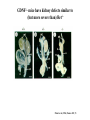
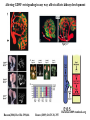
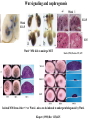
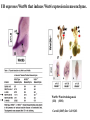
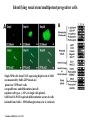
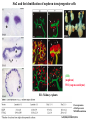
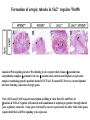
Kidney Development What does a mature kidney look like? How does it develop? Transcription factors required for kidney development : Wt1 & others Signaling pathways involved: GDNF-ret & Wnt Nephron stem/progenitor cells Chris Campbell [email protected] 829-3462 Oct 28, 2014 Renal capsule Cross section of a human kidney Functions of the kidney The functional unit of the kidney is the nephron ~13,000 in mice ~1,000,000 in humans Davidson 2009 The functional unit of the kidney is the nephron Quaggin (2008) Devt 135, 609 Human Anatomy, 3rd edition, 2001 Kidney development begins with specification of the intermediate mesoderm /Odd1 Dressler G R Development 2009;136:3863-3874 Development of the Kidney • The nephric duct and nephrogenic cord arise from intermediate mesoderm D>V • The nephric duct begins to elongate and undergo epithelialization in a rostral – caudal direction. Costantini (2010) Dev Cell 18, 698 Pax2 & Pax8 are required for the formation of the nephric duct ~E8.0 Laminin E-cadherin Bouchard Genes & Dev. 16,2958 (2002) Development of the Pronephros As this duct extends caudally (eventually joining up with the cloaca), the anterior region of the duct induces the adjacent mesenchyme (NC) to form the simple tubule(s) of the pronephros . •In mouse the pronephros consists of nothing more than a few cell condensates and is non functional but in amphibians such as Xenopus it is a functional kidney. Chan (2006) Exp Neph 103 e81 • In mammals, pronephric tubules and the anterior portion of the nephric duct degenerate, but the more caudal portions of the duct persist and serve as the Wolffian duct. Development of the Mesonephros • (B) As the pronephric tubules degenerate, the middle portion of the nephric duct induces a new set of kidney tubules in the mesenchyme constituting the mesonephros or mesonephric kidney. Sainio et al. (1997) Dev 124, 1293 Grote (2006) Dev 133, 53 Development of the Metanephros Bouchard (2004) Differentiation 72, 295 • (C) In male mammals, some of the mesonephric tubules persist as the vas deferens and efferent ducts of the testes but the remainder degenerates. • The metanephric kidney is initiated by the outgrowth of the ureteric bud from the Wolffian duct into the metanephric mesenchyme. ~E10.5 Development of the Metanephros • • • • • • • Shah et al (2004) Development 131, 1449 The development of the metanephros begins with the outgrowth of the ureteric bud from the Wolfian duct. The ureteric bud grows out into the metanephric mesenchyme which then condenses around the bud to form the metanephric blastema or mesenchyme. As this mesenchyme differentiates it induces the ureteric bud to branch and grow. At the tips of the branches the mesenchyme undergoes epithelialization to form the structures of the nephron The differentiated metanephric mesenchyme gives rise to the cells of the proximal and distal tubules, as well as the glomerular podocytes. Metanephric mesenchyme also gives rise to the renal stroma. This process of branching of the UB and differentiation of the mesenchyme continues along a radial axis until ~P2-4 with the earliest nephrons located inside and the newest nephrons in the periphery or nephrogenic zone. The ureteric bud gives rise to the ureter, the renal pelvis, and the collecting duct system. Branching of the ureteric bud Watanabe 2004 Bohnenpoll 2014 Kidney maturation involves reorientation within the body (Foxd1-/-) Levinson (2005) Dev 132, 529 Foxd1 Pax2 Hatini (1996) Gen&Dev 10, 1467 Transcription factors and Metanephric Development WT1 • WT1 was originally identified as a gene involved in Wilms tumor, a pediatric cancer in which kidney elements are incompletely differentiated and proliferate to form tumors. • Wt1 is first expressed in intermediate mesoderm prior to kidney development, and then in the nephrogenic cord and MM but not the WD or UB. The Wt1-/- Mouse E14.5 no kidney kidney E11.5 Wt1+/+ Wt1-/- In the absence of WT1 the UB fails to grow out from the Wolfian duct. Kreidberg et al. (1993) Cell 74, 679 Using in vitro organ culture to study the defect in WT1-/- mice Explanted murine kidney buds will partially differentiate in culture. Ureteric bud and metanephric mesenchyme can be mechanically or enzymatically separated and then recombined. When separated both tissues undergo apoptosis but if recombined UB will branch and MM will differentiate into tubules Inducer Responder Induction Positive Control Wt1+/+ WD or UB 18/23 Negative Controls Wt1+/+ WD Wt1+/+ Metanephric mesenchyme none Wt1+/+ Metanephric mesenchyme Wt1-/Metanephric mesenchyme Wt1+/+ Metanephric mesenchyme 3/42 none Experiment Wt1+/+ WD or UB Wt1-/- WD of MM differentiation 1/9 0/19 24/33 The Wolffian duct in Wt1-/- mice is normal and can induce a wild type metanephric mesenchyme to differentiate normally but the mesenchyme in Wt1-/- mice can neither signal to the Wolffian duct to form a UB nor can it respond to signals from the UB. Donovan et al.(1999) Dev. Genet. 24, 252 At what stage in MM differentiaton are Wt1-/- mice defective ? Pax2 E11 -11.5 Donovan et al.(1999) Dev. Genet. 24, 252 Six2 Conclusion Even though Wt1 is expressed in the intermediate mesoderm prior to formation of the metanephric mesenchyme, the earliest stages of metanephric mesenchyme differentiation don’t require Wt1 expression (or contact with UB). Other transcription factors involved in kidney development Eya1 (MM) Xu (1999) Nat Gen 23, 113 Sall1 (MM) E11.5 Nishinakamura (2001) Dev 128, 3105 Six1 (MM) UB forms but fails to branch. Can be rescued in vitro with Grem1 (antagonist of BMP signaling). Nie (2011) Dev Bio 352, 141 Emx2 (UB) E11.5 Miyamoto (1997) Dev 124, 1653 Signaling Pathways and Kidney Development Osr1 Ribes et al. J Am Soc Nephrol 14:S9, 2003 GDNF- Ret Signaling Majumdar (2003) Dev 130, 3175 • Ret was initially identified based on the ability of an oncogenic variant of the gene to transform NIH3T3 cells. • Sequence homology with other proteins identified ret as a receptor tyrosine kinase • Ret is expressed in the Wolfian duct and the ureteric bud. By the time the bud has branched several times, expression is restricted to the tips of the branches. Analyzing kidney development in Ret-/- Mice E11.5 +/+ E11.5 -/- • The phenotype of Ret-/- kidneys is variable ranging from complete absence of both kidneys and ureters to presence of two very small kidneys and relatively normal looking ureters. Schuchardt Nature 367,380 (1994) & Schuchardt (1996) Dev 122, 1919 Analysis of Ret-/- kidneys Conclusion (i)Mutant mesenchyme can signal wild type ureteric bud (ii) mutant ureteric bud cannot respond to wt mesenchyme. THE DEFECT IS IN THE URETERIC BUD. Schuchardt et al. (1996) Development 122, 1919 Identifying the ret receptor ligand GDNF was first identified as a factor capable of promoting growth of neurons in culture Following cloning of the gene, its expression pattern (expressed in MM but not UB) suggested it might play a role in kidney development. Mouse kidney organ culture + Conditioned medium from cells expressing GDNF control +aGDNF Ab +recombinant human GDNF Vega et al. (1996) PNAS 93, 10657 GDNF-/- mice have kidney defects similar to (but more severe than) Ret-/- Pichel et al. (1996) Nature 382, 73 Altering GDNF-ret signaling in any way affects affects kidney development Spry1-/- Basson(2006) Dev Bio 299,466 Rozen (2009) JASN 20, 255 Davidson 2009 stembook.org Wnt signaling and nephrogenesis Wnt4 E12.5 Wnt4 E11.5 E15 Wnt4-/- MM fails to undergo MET Stark (1994) Nature 372, 679 Isolated MM from either +/+ or Wnt4-/- mice can be induced to undergo tubulogenesis by Wnt4. Kispert (1998) Dev 125,4425 UB expresses Wnt9b that induces Wnt4 expression in mesenchyme. + Wnt9b>Wnt4>tubulogenesis (UB) (MM) Carroll (2005) Dev Cell 9,283 Identifying renal stem/multipotent progenitor cells Single MM cells from E11.5 expressing high levels of Sall1 (as measured by Sall1-GFP knock-in) plated on 3T3Wnt4+ cells can proliferate and differentiate into all nephron cell types. (~10% of single cells plated). Sall1 itself is NOT required (differentiation occurs in cells isolated from Sall1-/- MM although colony size is reduced). Osafune (2006) Dev 133, 151 Six2 and the identification of nephron stem/progenitor cells (UB) (nephron) Wt1 (cap mesenchyme) E11.5 kidney explants Overexpression of Six2 prevents MM differentiation Self(2006) EMBO25,5214 Formation of ectopic tubules in Six2-/- requires Wnt9b Canonical Wnt signaling involves Wnt binding to its receptor which releases b-catenin from a degradation complex. b-catenin levels rise, b-catenin enters nucleus and displaces a repression complex (containing groucho proteins) bound to TCF/Lef. B-catenin/TCF acts as a transcriptional activator inducing expression of target genes. Park (2012) used Ch-IP/seq and transcription profiling to show that Six2 and Wnt (via b-catenin & Tcf/Lef) regulate self-renewal and commitment of nephron progenitors through shared gene regulatory networks. Some genes activated by one are repressed by the other while other genes require both Six2 and Wnt signaling to be expressed. Main conclusion(s): What is the next experiment?










![Urinary System_student handout[1].](http://s1.studyres.com/store/data/008293858_1-b77b303d5bfb3ec35a6e80f57f440bef-150x150.png)









