* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Soul Mate Real Time, Non-Invasive Heart Data
Remote ischemic conditioning wikipedia , lookup
Jatene procedure wikipedia , lookup
Heart failure wikipedia , lookup
Cardiothoracic surgery wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Coronary artery disease wikipedia , lookup
Electrocardiography wikipedia , lookup
Heart arrhythmia wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
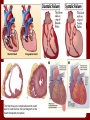
Soul Mate Real Time, Non-Invasive Heart Data Anthony Sierra Christine Cherop Onyebuchi Udozorh Outline • Physiology and pathophysiology of the condition to be treated • Prior treatments/therapies • Therapeutic procedures-complete protocols • Cost and Coverage • Expected post-therapy improvement • Expected post-therapy complications • Why is Soul Mate the best choice? Physiology and Pathophysiology: Heart Failure and Transplant Rejection • • • • • • • Heart transplants are typically performed on patients with end-stage heart failure or patients with severe coronary heart disease Heart failure occurs when the heart is incapable of maintaining a cardiac output that is adequate to meet metabolic requirements and accommodate venous return. Coronary heart disease is caused by plaque building up along the inner walls of the arteries, making the arteries narrower hence restricting blood flow to the heart After a heart transplant, a patient returns to the hospital for regular checkups and remains on immunosuppressants for the rest of his/her life Acute and chronic allograft rejections are the most significant factors limiting heart transplants Regular monitoring is required to detect rejection, and is normally done by regular biopsy that is later replaced by AlloMap Molecular Expression Testing, a gene expression blood test The first three are complications that could lead to heart failure, the last diagram is the heart transplant procedure Prior Treatments/Therapies • • • Endomyocardial biopsies with examination of structural damage or increased levels of T cells Identification of patient symptoms of potential rejection Traditional pacemakers/defibrillators without additional detection function Monitoring the heart, posttransplant • • • The functional state of the heart can be closely monitored by the heart's electrical activity Analysis of intramyocardial electrocardiography (IMEG) is considered sensitive and specific for allograft rejection The peak to peak amplitude (PPA) of the unipolar IMEG has been shown sensitive to a variety of alterations in myocardial physiology Soul Mate- • • • Soul mate, an Implantable Heart Transplant rejection monitor is based on a new technology- Cardiac Rejection Device that senses naturally occurring electrical activity of the heart and automatically applies electrical stimulation treatment if needed The device is still on trial, and TransWorld hopes to get FDA approval at the end of the trial Phase This is the second generation of the Soul Mate. The first version, designed for patients following a heart transplant, is being marketed in Europe. How it works • • • The components of the Soul Mate Heart Transplant Monitoring system, Horai T et al. Circulation; 2009, 120:S185- S190 • • • • Nine IMEG parameters recorded The Cardiac Rejection Monitoring Device (CRD), non-invasively implanted under the skin, records IMEG through standard 3 leads at programmable times in either bipolar or unipolar configurations. 1 lead is placed on the epicardial surface of the right ventricle and the other two on the left ventricle Information of 9 IMEG parameters from QRS complexes are recorded and later analyzed Onelife wand is held over the Cardiac Rejection Monitoring Device to transfer data Data transferred to Home Call Box through the wireless bluetooth connection Data is then sent automatically to the Central Monitoring Centre for analysis Costs and Coverage • • • • No current costs/insurance coverage information on Soul Mate Current pacemaker and surgery cost: Dependent on type of pacemaker, length of stay, location ~ $19,651 total in Ohio, $28,348 in Western United States (without insurance) Covered by Medicare and private insurance: costs between 2.5-4k Expected Post-Therapy Improvements "This device would potentially reduce the number of biopsies needed and result in earlier detection and treatment of rejection. It is possible that the device could actually replace EMB and allow vastly more frequent allograft rejection assessment that would assist the clinician with day-to-day, evidence-based adjustments of complicated and toxic immunosuppression cocktails. Furthermore, using the transtelephonic measurements could be beneficial for patients who can be monitored at great distances from the transplant center. There is a potential that the Soul Mate system would offer a method for less invasive and more effective management of HTx patients"- Horiai etal • Horai et.al found that Soulmate, when used in Cannine models, has the potential to non-invasively detect allograft rejection and reduce the number of biopsies. Expected Post-Therapy Complications Due to still being in development phase, we are unable to find concrete information on expected post-therapy complications. However, based on complications with the typical pacemaker and surgery in general, we can infer a variety of potential complications: • Lead Displacement • Tissue Scarring • Heating of the Cardiac Tissue • Fibrosis • "Twiddler's Syndrome" • Infection (Surgical Site) Why Is the Soul Mate the Best Choice? • • • • • • • The Soul Mate can detect allograft rejection earlier than the typical methods. Non-invasive Reduces the number of endomyocardial biopsies needed for detection, and could actually replace biopsies Very accurate Allows for 24/7 monitoring in and out of the hospital- cutting costs arising from unnecessary doctors visits Pacemaker or defibrillator capabilities Prolong morbidity free survival Reference "Cost of a Pacemaker." CostHelper. CostHelper, Inc., n.d. Web. 29 Apr. 2013. Horai et al. "Novel Implantable Device to Detect Cardiac Allograft Rejection." Circulation 120 (2009): 185190. AHAJournals. Web. 29 Apr. 2013. Thomas, Jennifer. "Cardiac device maker Transworld to launch $25M clinical trial." Charlotte Business Journal. American City Business Journals, 23 Mar. 2012. Web. 29 Apr. 2013.