* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The Florigen Genes FT and TSF Modulate
Plant tolerance to herbivory wikipedia , lookup
Plant stress measurement wikipedia , lookup
History of herbalism wikipedia , lookup
Plant secondary metabolism wikipedia , lookup
Historia Plantarum (Theophrastus) wikipedia , lookup
Plant nutrition wikipedia , lookup
Plant defense against herbivory wikipedia , lookup
History of botany wikipedia , lookup
Arabidopsis thaliana wikipedia , lookup
Plant use of endophytic fungi in defense wikipedia , lookup
Venus flytrap wikipedia , lookup
Ornamental bulbous plant wikipedia , lookup
Evolutionary history of plants wikipedia , lookup
Plant breeding wikipedia , lookup
Plant physiology wikipedia , lookup
Plant ecology wikipedia , lookup
Plant reproduction wikipedia , lookup
Sustainable landscaping wikipedia , lookup
Plant morphology wikipedia , lookup
Flowering plant wikipedia , lookup
Glossary of plant morphology wikipedia , lookup
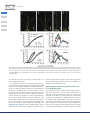
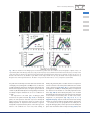
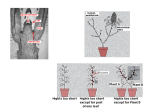
Special Focus Issue – Regular Paper The Florigen Genes FT and TSF Modulate Lateral Shoot Outgrowth in Arabidopsis thaliana Kazuhisa Hiraoka1, Ayako Yamaguchi1, Mitsutomo Abe1,2 and Takashi Araki1,* 1 Division of Integrated Life Science, Graduate School of Biostudies, Kyoto University, Yoshida-Konoe-cho, Sakyo-ku, Kyoto, 606-8501 Japan 2 Department of Biological Sciences, Graduate School of Science, The University of Tokyo, 7-3-1, Hongo, Bunkyo-ku, 113-0033 Japan *Corresponding author: E-mail, [email protected]; Fax, +81-75-753-6470. (Received October 4, 2012; Accepted November 27, 2012) Successful sexual reproduction of a plant with prolific seed production requires appropriate timing of flowering and concomitant change of architecture (e.g. internode elongation and branching) to facilitate production of the optimal number of flowers while enabling continued resource production through photosynthesis. Florigen is the prime candidate for a signal linking the two processes. Growth analysis of lateral shoots in mutants of FLOWERING LOCUS T (FT) and TWIN SISTER OF FT (TSF) revealed a delay in the onset of outgrowth and a reduction of the growth rate in ft plants in long-day (LD) conditions and in tsf plants in short-day (SD) conditions. Thus, as in the case of floral transition, FT and TSF play dominant roles in LD and SD conditions, respectively, in the promotion of lateral shoot development. Differential expression patterns of the two genes were in good agreement with their differential roles both in the floral transition and in lateral shoot development under contrasting photoperiod conditions. By manipulating florigen production after bolting of the primary shoot, it was shown that florigen promotes lateral shoot growth independently of its effect on the floral transition of the primary shoot. Analysis of growth and gene expression in lateral shoots of the mutants suggests that the loss of florigen leads to a reduced rate of flower formation on lateral shoots. Together, we propose that the two florigen genes are an important key to linking the floral transition and lateral shoot development to maximize the reproductive success of a plant. Keywords: Arabidopsis Branching Florigen FT (FLOWERING LOCUS T) Lateral shoot outgrowth TSF (TWIN SISTER OF FT). Abbreviations: ACT2, ACTIN2; AP1, APETALA1; Col, Columbia; CYCD, CYCLIN D; CK, cytokinin; DAS, days after sowing; FLC, FLOWERING LOCUS C; FT, FLOWERING LOCUS T; FUL, FRUITFULL; GA20ox, GIBBERELLIN 20-OXIDASE; GUS, b-glucuronidase; HSP, HEAT SHOCK PROTEIN 18.2; Ler, Landsberg erecta; LFY, LEAFY; LD, long day; No, Nossen; NRQ, normalized relative quantitiy; PEBP, phosphatidylethanolamine-binding protein; PIN, PINFORMED; RT–PCR, reverse transcription–PCR; RT–qPCR, reverse transcription–quantitative real-time PCR; STM, SHOOT MERISTEMLESS; SD, short day; SAM, shoot apical meristem; SFT, SINGLE FLOWER TRUSS; SOC1, SUPPRESSOR OF OVEREXPRESSION OF CO1; TSF, TWIN SISTER OF FT; WUS, WUSCHEL; ZT, Zeitgeber time Introduction In flowering plants, successful sexual reproduction leading to prolific seed production requires appropriate timing of flowering. In annual plants such as Arabidopsis, also important is appropriate remodeling of the plant architecture (e.g. internode elongation and branching) to facilitate production of the optimal number of flowers while enabling continued resource production through photosynthesis for seed formation. Therefore, a tight link between the floral transition and the concomitant architectural change should somehow be achieved. A systemic signaling mechanism will be the prime candidate for the regulatory basis of linking the two processes. In regulation of the timing of the floral transition, the environmental cues (photoperiod, light quality and quantity, temperature, and water and nutrient availability) and the internal cues (age, size and phytohormone dynamics) converge on the ‘hubs’ known as the floral pathway integrator genes (Levy and Dean 1998, Araki 2000, Srikanth and Schmid 2011, Yamaguchi and Abe 2012). In Arabidopsis, the pathways responsive to the appropriate environmental conditions (photoperiod and vernalization) and those which reflect the internal status of plants (autonomous, gibberellin and aging) intricately regulate the four integrator genes. Among them, FLOWERING LOCUS T (FT) and its paralog TWIN SISTER OF FT (TSF) encode approximately 20 kDa globular proteins of the Plant Cell Physiol. 54(3): 352–368 (2013) doi:10.1093/pcp/pcs168, available online at www.pcp.oxfordjournals.org ! The Author 2012. Published by Oxford University Press on behalf of Japanese Society of Plant Physiologists. All rights reserved. For permissions, please email: [email protected] 352 Plant Cell Physiol. 54(3): 352–368 (2013) doi:10.1093/pcp/pcs168 ! The Author 2012. Role of FT and TSF beyond flowering phosphatidylethanolamine-binding protein (PEBP) family (Kardailsky et al. 1999, Kobayashi et al. 1999). In young plants, long-day (LD) photoperiods up-regulate their expression in phloem companion cells of cotyledons and leaves (FT and TSF) and hypocotyl (TSF), and the autonomous and vernalization pathways negatively regulate both of the genes (Suárez-López et al. 2001, Yanovsky and Kay 2002, Takada and Goto 2003, Michaels et al. 2005, Yamaguchi et al. 2005). As systemic long-distance signaling molecules called florigen, FT and TSF proteins are transported to the shoot apical meristems (SAMs) (Corbesier et al. 2007, Jaeger and Wigge 2007, Mathieu et al. 2007, Notaguchi et al. 2008), where they form a complex involving a bZIP transcription factor FD to up-regulate the expression of MADS-box genes such as APETALA1 (AP1), which then orchestrates floral initiation and development (Abe et al. 2005, Wigge et al. 2005, Kaufmann et al. 2010). In rice, and possibly in Arabidopsis as well, formation of the florigen complex is mediated by 14-3-3 proteins (Purwestri et al. 2009, Taoka et al. 2011). Of the remaining two integrator genes, a MADS-box gene, SUPPRESSOR OF OVEREXPRESSION OF CO1 (SOC1), is involved in floral induction and commitment, flower organ formation, meristem determinacy, and prevention of secondary growth and shoot longevity (Melzer et al. 2008, Liu et al. 2009, Lee and Lee 2010, Torti et al. 2012). The fourth integrator gene LEAFY (LFY), which encodes a plant-specific transcription factor, also plays multifaceted roles in floral competency, establishment and maintenance of inflorescence and floral meristem identities, and floral organ formation (Moyroud et al. 2010). Flowering in many species is generally accompanied by various changes including increased leaf growth rate and change in phyllotaxis (Bernier et al. 1981). As a rosette-forming plant, Arabidopsis shows increased internode elongation of the primary shoot axis (bolting) and initiation and outgrowth of lateral buds to develop a racemose inflorescence with complex lateral shoot systems. Of these changes, development of lateral shoot systems strengthens the reproductive success of the plant by increasing the number of flowers and the amount of photosynthetic tissues available for resource production. It has been shown that, as in the case of flowering, plants sense the environmental and internal cues to decide whether to allow outgrowth of each of their lateral buds (Domagalska and Leyser 2011). The central mechanism involved in the regulation of lateral shoot outgrowth is called correlative inhibition, in which each shoot including the main shoot competitively inhibits outgrowth of other shoots through each other’s polar auxin transport stream (Ongaro et al. 2008, Prusinkiewicz et al. 2009). Besides auxin, cytokinins (CKs) and strigolactones are involved in this mechanism (Tanaka et al. 2006, Hayward et al. 2009, Crawford et al. 2010, Minakuchi et al. 2010). Inside the buds, cell cycle re-progression is thought to be important (Shimizu and Mori 1998, Tatematsu et al. 2005, Müller and Leyser 2011). However, in spite of these advances in our understanding, the regulatory link between flowering and lateral shoot development still remains to be investigated. Recent studies showed that the florigen genes in Arabidopsis and other plants play important roles in various physiological aspects other than flowering. In Arabidopsis, FT regulates stomatal opening (Kinoshita et al. 2011), meristem maintenance in cooperation with SHOOT MERISTEMLESS (STM) during inflorescence development (Smith et al. 2011), and prevention of indeterminate growth, floral reversion and aerial rosette formation (Melzer et al. 2008). FT is also implicated in FLCmediated regulation of seed germination (Chiang et al. 2009) and some traits of vegetative phase change (Willmann and Poethig 2011). In tomato, SINGLE FLOWER TRUSS (SFT) regulates reiterative growth of shoots and influences leaf maturation, compound leaf architecture, stem growth, and abscission zone formation (Shalit et al. 2009). Also, allelic variation at the SFT locus is implicated in heterosis for yield (Krieger et al. 2010). In rice, Heading date1, encoding a CONSTANS ortholog, and Early heading date1, encoding a type-B response regulator unique to Poaceae, regulate spikelet number per panicle in concert through the up-regulation of two rice florigen genes Heading date3a (Hd3a) and Rice Flowering-locus T1 (Endo-Higashi and Izawa 2011). Increased tillering was observed in transgenic rice plants expressing green fluorescent protein-fused Hd3a under the phloem-specific promoters, as was accelerated flowering (Tamaki et al. 2007). In a potato cultivar which tuberizes only in short-day (SD) conditions, overexpression of rice Hd3a induced flowering and tuberization in non-inductive LD conditions (Navarro et al. 2011). The potato genome contains nine PEBP family genes, of which StSP3D acts as florigen, while StSP6A acts as ‘tuberigen’. In Populus trees, SD-induced growth cessation and bud set in autumn involve the regulation of PtFT1 (Böhlenius et al. 2006). Thus, there emerged a view that FT proteins act as universal long-distance systemic signals mainly to regulate shoot meristem activities. In this study, we investigated the roles of the two florigen genes, FT and TSF, in the regulation of branch outgrowth to uncover the regulatory link between flowering and lateral shoot development in Arabidopsis. A comparison among triple and quadruple mutants of the four floral pathway integrator genes clearly showed that a defect in florigen genes causes retardation of lateral shoot outgrowth. Then, detailed growth analyses of lateral shoots in single and double mutants revealed the delay in outgrowth onset and the reduction of growth rate in ft plants under LD conditions and in tsf plants under SD conditions. Thus, as in the case of the floral transition, FT and TSF play dominant roles in LD and SD, respectively, in the promotion of lateral shoot development. Differential patterns and profiles of expression of the two genes revealed in this study were in good agreement with their differential roles both in the floral transition and in lateral shoot development under contrasting photoperiod conditions. By employing daylength shifts and an inducible FT transgene to manipulate florigen production after the bolting of the primary shoot, the role of florigen in promoting lateral shoot growth independently of its effect on the floral transition of the primary shoot was clearly Plant Cell Physiol. 54(3): 352–368 (2013) doi:10.1093/pcp/pcs168 ! The Author 2012. 353 K. Hiraoka et al. demonstrated. A delay in the first flower anthesis and a reduced rate of flower production as well as reduced expression of some genes involved in flower development were observed in lateral shoots of the mutants. These observations may suggest a hypothesis to be tested in the future that the two florigen genes may regulate lateral shoot outgrowth and elongation via acceleration of flower formation. Taken together, we propose that the two florigen genes are an important key to linking the floral transition and lateral shoot development to maximize the reproductive success of a plant. Results Retardation of lateral shoot elongation caused by loss-of-function mutations of FT and TSF During the course of study of the four floral pathway integrator genes, ft-1; tsf-1; lfy-1; soc1-2 quadruple mutants were generated. Under LD photoperiods, unlike the previously reported co; ga1; fca triple mutants which neither bolted nor flowered without vernalization (Reeves and Coupland 2001), the quadruple mutants eventually bolted like ft; lfy double (Ruiz-Garcı́a et al. 1997) and ft; soc1; lfy triple (Moon et al. 2005) mutants. However, they never produced organs with flower-like characteristics, such as stigmatic papillae, which eventually appeared in ft; lfy double and ft; soc1; lfy triple mutants (Ruiz-Garcı́a et al. 1997; data not shown). Intriguingly, the quadruple mutants showed severe growth retardation of the secondary and tertiary lateral shoots (Fig. 1A, D), resulting in a much simpler appearance than the bushy phenotype of the ft; lfy double mutants (see Ruiz-Garcı́a et al. 1997). Whereas the absence of the LFY function occasionally resulted in an empty axil phenotype, the above-mentioned simple appearance was observed even in the presence of the wild-type LFY allele (Fig. 1E). In contrast, the presence of the wild-type TSF allele and, to some extent, the wild-type SOC1 allele did recover the growth of secondary and tertiary lateral shoots (Fig. 1B–D). The observed effect of SOC1 on lateral shoot development is consistent with recent reports (Melzer et al. 2008, Dorca-Fornell et al. 2011). However, the effect of TSF was more drastic than that of SOC1, implying that the two florigen genes have hitherto unexplored roles in lateral shoot growth in Arabidopsis. Based on these observations, the role of FT and TSF in lateral shoot growth was examined in more detail. Because few axillary shoots showed consistent vigorous outgrowth from the rosette leaf axils of LD-grown plants carrying the defective ft mutation and SD-grown plants (Supplementary Fig. S1; data not shown), our analysis mainly focused on the lateral shoots formed at the cauline leaf axils on the primary inflorescence stem. When growth of the lateral shoot from the cauline leaf axil was compared among the various nodes along the stem, a similar tendency was observed (Supplementary Fig. S1). Therefore, the longest lateral shoot (for definition, see the Materials and Methods) was chosen as a representative example hereafter and designated simply as ‘lateral shoot’. 354 Single and double mutants (ft-2, tsf-1 and ft-2; tsf-1) as well as their wild-type plants [accession Columbia (Col)] were grown under inductive LD or non-inductive SD conditions, and the main and lateral shoot lengths were measured on a daily basis. ‘Day 0’ was defined as the day on which the main shoot of a plant reached a height of 5 cm. The first flower of the main shoot was at anthesis around Day 0 under our growth conditions. LD-grown wild-type and tsf-1 plants and SD-grown wild-type plants ceased producing flowers around Day 14 and Day 21, respectively, under our growth conditions. Therefore, shoot lengths were measured until Day 14 for LD-grown wild-type and single mutant plants, until Day 21 for SD-grown wild-type plants, and until Day 28 for LD-grown double mutants and SD-grown single and double mutants. As explained above, the lengths of the longest lateral shoots were compared among the four tested lines. Under LD conditions, wild-type and tsf-1 plants showed well-grown main and lateral shoots (Fig. 2A, B, E, G), as expected from their flowering phenotypes (Supplementary Table S1; Michaels et al. 2005, Yamaguchi et al. 2005, Jang et al. 2009). However, ft-2 plants showed retarded growth of lateral shoots with well-grown main shoots, and ft-2; tsf-1 double mutants showed more drastic retardation of lateral shoot growth (Fig. 2C–E, G). To analyze this lateral shoot phenotype in ft; tsf double and ft single mutants in detail, the elongation rate of the lateral shoots (for definition, see the Materials and Methods) was calculated. As shown in Fig. 2H, the retardation phenotype was characterized by two components: the delay in outgrowth initiation and the slower elongation rate. Moreover, the delay in the onset of outgrowth was accompanied by the delayed timing of first flower anthesis and a reduced rate of flower formation on the lateral shoots (Fig. 2F). Under SD conditions, wild-type and ft-2 plants showed similar main and lateral shoot growth (Fig. 3A, C, F, H, I). Surprisingly, tsf-1 plants showed apparent retardation of lateral shoot growth compared with wild-type and ft-2 plants (Fig. 3A–C, H, I). ft-2; tsf-1 plants showed more drastic retardation of lateral shoot growth, like those grown under LD conditions (Fig. 3D, H, I). tsf-1 and ft-2; tsf-1 plants sometimes developed aerial rosettes at the axil of lower cauline leaves even on Day 7 (Fig. 3E). This is consistent with the suggested role of FT in inhibition of aerial rosette formation in concert with SOC1 and FRUITFULL (FUL) (Melzer et al. 2008). As in the case of LD conditions, a delay in anthesis of the first flower and a reduced flower formation rate were observed on the lateral shoots of tsf-1 and ft-2; tsf-1 mutants under SD conditions (Fig. 3G). Next, we utilized ft-2 and ft-2; tsf-1 plants in accession Landsberg erecta (Ler) and ft-4, ft-5 and ft-6 plants in accession Nossen (No) to examine the effect of various ft alleles and genetic backgrounds. LD-grown ft-2 and ft; tsf plants in the Ler background (Fig. 4A, C, E) and various ft mutants in the No background (Fig. 4B, D, F) showed a phenotype similar to that of LD-grown ft-2 and ft-2; tsf-1 plants in the Col background (Fig. 2). Therefore, we conclude that the effect of Plant Cell Physiol. 54(3): 352–368 (2013) doi:10.1093/pcp/pcs168 ! The Author 2012. Role of FT and TSF beyond flowering Fig. 1 Branching phenotype of triple and quadruple combinations of ft, tsf, soc1 and lfy mutations. The plants were grown under LD conditions. (A) An ft-1; tsf-1; soc1-2; lfy-1 quadruple mutant at 124 DAS (days after sowing). (B) An ft-1; tsf-1; lfy-1 triple mutant at 124 DAS. (C) An ft-1; soc1-2; lfy-1 triple mutant at 124 DAS. (D) An ft-1; soc1-2; lfy-1 triple mutant (left) and an ft-1; tsf-1; soc1-2; lfy-1 quadruple mutant (right) at 98 DAS. (E) The effect of a strong lfy mutation in the ft; tsf; soc1 background. A lfy-1 homozygous plant (quadruple (lfy); left), a lfy-1 heterozygous plant (LFY/lfy; center) and a wild-type homozygous plant (LFY+; right), all in the ft-1; tsf-1; soc1-2 background at 83 DAS, are shown. Arrowheads indicate axils devoid of a lateral bud. Scale bars: (A–C) 10 cm; (D) 5 cm; (E) 1 cm. Plant Cell Physiol. 54(3): 352–368 (2013) doi:10.1093/pcp/pcs168 ! The Author 2012. 355 K. Hiraoka et al. Fig. 2 Effect of mutations in the florigen genes on lateral shoot growth under LD conditions. (A–D) Photographs of plants on Day 7 (Day 0 was defined as the date when the main stem reached 5 cm height). A representative plant of the wild type (Col) (A), tsf-1 (B), ft-2 (C) and ft-2; tsf-1 (D). Scale bars: 5 cm. (E) Growth of the main shoot. (F) Number of open flowers on the longest lateral shoot per day. (G) Growth of the longest lateral shoot. (H) Elongation rate of the longest lateral shoot. The data in E–H represent the mean ± SD (n = 7 to 9), except for F (mean ± SEM). loss-of-function ft and/or tsf mutations is neither allele- nor genetic background-specific. The expression of FT and TSF in young SD-grown plants is much lower than in LD-grown plants (Yanovsky and Kay 2002, Cerdán and Chory 2003, Yamaguchi et al. 2005; see Fig. 6), and the LD to SD shift attenuates FT expression in 2-week-old plants (Corbesier et al. 2007, Osnato et al. 2012). Therefore, we expected that the LD to SD shift resulted in rapid reduction in the available amount of FT and TSF proteins followed by the decrement of lateral shoot growth. To test this, we grew wild-type (Col) and tsf-1 plants under LD conditions until their main shoot length exceeded 2 or 5 cm, and then moved batches of plants to SD conditions. As expected, LD-grown wild-type and tsf-1 plants showed no obvious difference in flowering time (Supplementary Table S1), while SD-shifted plants showed reduced lateral and main shoot growth irrespective of tsf-1 356 mutation (Fig. 5). Taken together, these results suggest that the two florigen genes promote lateral shoot outgrowth: FT mainly in LD and TSF mainly in SD. Spatio-temporal expression patterns of TSF and FT in SD-grown plants The observation of SD-grown plants (Fig. 3) suggests that TSF plays a greater role than FT in the promotion of lateral shoot outgrowth under SD conditions. However, the expression of TSF under SD conditions in mature plants has not been well investigated (cf. Yamaguchi et al. 2005). Therefore, we observed the temporal and spatial profiles of TSF and FT expression under SD conditions using reverse transcription–quantitative PCR (RT–qPCR) and semi-quantitative reverse transcription– PCR (RT–PCR) techniques and GUS (b-glucuronidase) staining of gTSF::GUS and gFT::GUS lines. In both LD and SD conditions, Plant Cell Physiol. 54(3): 352–368 (2013) doi:10.1093/pcp/pcs168 ! The Author 2012. Role of FT and TSF beyond flowering Fig. 3 Effect of mutations in the florigen genes on lateral shoot growth under SD conditions. (A–D) Photographs of plants on Day 7. A representative plant of the wild type (Col) (A), tsf-1 (B), ft-2 (C) and ft-2; tsf-1 (D). (E) Aerial rosette formation observed in ft-2; tsf-1 on Day 7. Parts of the same plant are shown in the upper and lower photographs. Arrowheads indicate aerial rosettes. Scale bars: (A–D) 5 cm; (E) 1 cm. (F) Growth of the main shoot. (G) Number of open flowers on the longest lateral shoot per day. (H) Growth of the longest lateral shoot. (I) Elongation rate of the longest lateral shoot. The data in F–I represent the mean ± SD (n = 8 or 9), except for G (mean ± SEM). the peak of FT and TSF expression was observed around the end of the light period (Yamaguchi et al. 2005). Hence, to detect the possible peak levels of expression, aerial parts of the plants were harvested at Zeitgeber time (ZT) 7 for plants grown under SD conditions [20, 40 and 60 days after sowing (DAS)] and at ZT15 for 60-day-old plants shifted from SD to LD conditions on 57 DAS. TSF expression in the aerial parts of wild-type plants increased as the plants matured (Fig. 6A, B). In 20-day-old plants, the TSF expression level was very low. In 40-day-old plants, however, TSF expression started to increase, and in 60-day-old plants its expression level increased to approximately 80% compared with the plants of the same age transferred to LD conditions for 4 d. In fact, GUS staining of 20-day-old gTSF::GUS plants revealed that the TSF expression site was restricted to the vicinity of the SAM and to the vascular tissues of hypocotyls (Fig. 6D, F, H, J), as reported previously (Yamaguchi et al. 2005). In 40-day-old plants, TSF expression was detected in the midvein of some fully expanded rosette leaves (Fig. 6M, N) and in 60-day-old plants, some of which already bolted in this experiment, TSF expression was detected in the midvein and secondary veins of older rosette leaves, irrespective of photoperiod (Fig. 6Q, R, U, V, Y, Z; Supplementary Fig. S3). The results from these GUS staining analyses are consistent with the RT–PCR and RT–qPCR results. In contrast, FT was not up-regulated in the aerial parts of SD-grown wild-type plants, and its expression in the 60-day-old plants was much lower than TSF expression (Fig. 6A, B). GUS Plant Cell Physiol. 54(3): 352–368 (2013) doi:10.1093/pcp/pcs168 ! The Author 2012. 357 K. Hiraoka et al. Fig. 4 Effect of various ft mutant alleles and genetic backgrounds. ft-2 and ft-2; tsf-1 in the Ler background (A, C, E) and ft-4, ft-5 and ft-6 in the No background (B, D, F) and their respective wild-type plants were used. (A, B) Growth of the main shoot. (C, D) Growth of the longest lateral shoot. (E, F) Elongation rate of the longest lateral shoot. The data represent the mean ± SD (n = 8 or 9). Fig. 5 Effect of LD to SD shift treatment on shoot growth. Lengths of the main shoot (A) and the longest lateral shoot (B) were measured on Day 7. The data represent the mean ± SD (n = 7 or 8). The double asterisks and the double daggers indicate P < 0.01 compared with the LD-grown wild-type (Col) plants and tsf-1 plants, respectively. stains of SD-grown gFT::GUS plants were faintly detected in the tertiary vein of older rosette leaves (Fig. 6C, E, G, I, K, L, P, T, X; Supplementary Fig. S2B). Daylength shift to LDs caused the 60-day-old plants to up-regulate the FT expression strongly, 358 mainly in the tertiary veins (Fig. 6A, B, O, S, W; Supplementary Fig. S2A). Thus, in our growth conditions, FT expression in rosette leaves was regulated largely by photoperiod, while TSF expression in leaves was regulated largely in an age-dependent manner. It is to be noted that a much higher expression level of TSF than that of FT in leaves in SD is accountable for its greater role in the lateral shoot growth in this photoperiod condition. The spatial expression pattern of FT and TSF after floral transition was further investigated with SD-grown or SD to LD-transferred gFT::GUS and gTSF::GUS plants. The expression patterns in the cauline leaves were basically similar to those in the rosette leaves (especially younger rosette leaves, Fig. 7I–L); FT expression in the tertiary veins of cauline leaves was LDdependent (compare Fig. 7E, I with Fig. 7F, J), and TSF expression in the main vein tended to be observed in cauline leaves at lower nodes (see Fig. 7L). No or faint signals were detected in the apical regions of lateral shoots which were approximately 0.5–1 cm long (Fig. 7E–H). However, in developed inflorescences, FT expression was increased in the veins of pedicels approximately from floral stage 11 (defined by Smyth et al. Plant Cell Physiol. 54(3): 352–368 (2013) doi:10.1093/pcp/pcs168 ! The Author 2012. Role of FT and TSF beyond flowering Fig. 6 Spatio-temporal expression patterns of FT and TSF in SD-grown plants. (A, B) Temporal profiles of FT and TSF expression in SD-grown wild-type (Col) plants. (A) RT–qPCR analyses of FT (left) and TSF (right) expression. The data represent the means of five biological replicates ± SD. (B) Relative comparison between FT and TSF expression levels through a modified RT–PCR analysis. Two of five cDNA samples per group used in the RT–qPCR analyses in A were used as templates for digestion with EcoT14I (a site in TSF) or PstI (a site in FT). PCR products of the full-length cDNA clones of FT and TSF were used as controls for digestion. EcoT14I-digested products (upper) and PstI-digested products (middle) were electrophoresed on agarose gels. ACT2 (lower) was used as a reference. (C–J) GUS staining patterns of a gFT::GUS plant (C, E, G, I) and a gTSF::GUS plant (D, F, H, J) grown under SD conditions at 20 DAS. (C, D) Hypocotyls and shoot apical regions. (E, F) Rosette leaves. (G, H) Enlarged views of the apical regions in C and D. (I, J) Enlargements of part of the leaves in E and F. The arrowheads in H and I indicate faint signals. (continued) Plant Cell Physiol. 54(3): 352–368 (2013) doi:10.1093/pcp/pcs168 ! The Author 2012. 359 K. Hiraoka et al. 1990) and in the medial vascular bundles and funiculus veins of styles at later stages in a photoperiod-independent manner, and only the veins of sepals showed photoperiod-dependent FT induction (Fig. 7A, B). In contrast, faint TSF signals were observed in the veins around receptacles from floral stage 12 (Fig. 7C, D). Thus, post-transitional expression of FT and TSF was also observed in veins under SD conditions, while their expression profiles in inflorescences were different from those in rosette and cauline leaves. Recovery from retardation of lateral shoot elongation by induced FT and TSF expression We then investigated whether recovery of FT and/or TSF function from their deficient states can rescue the outgrowth Fig. 7 Expression patterns of FT and TSF in the inflorescences of SD-grown plants. A gFT::GUS plant (A, E, I) and a gTSF::GUS plant (C, G, K) were transferred from SD to LD conditions at 57 DAS, while another gFT::GUS plant (B, F, J) and gTSF::GUS plant (D, H, L) were kept under SD conditions for 60 d. (A–D) Inflorescences of the main shoot. (E–H) Apical regions of the lateral shoots. (I–L) Cauline leaves and their nodal regions. Scale bars: (A–D, I–L) 2 mm; (E–H) 1 mm. Fig. 6 Continued Scale bars: (C, D) 1 mm; (E, F) 2 mm; (G, H) 200 mm; (I, J) 400 mm. (K–N) GUS staining patterns of a gFT::GUS plant (K, L) and a gTSF::GUS plant (M, N) grown under SD conditions at 40 DAS. (K, N) Whole aerial parts. (L, M) Enlargements of part of the leaves in K and N. The arrowheads in L and N indicate faint signals. Scale bars: (K, N) 1 cm; (L, M) 500 mm. (O–Z) Spatial expression patterns of FT and TSF in SD-grown plants at 60 DAS. A gFT::GUS plant (O, S, W) and a gTSF::GUS plant (Q, U, Y) were transferred from SD to LD conditions at 57 DAS, while another gFT::GUS plant (P, T, X) and gTSF::GUS plant (R, V, Z) were kept under SD conditions. (O–R) Immature rosette leaves. (S–V) Mature rosette leaves. (W–Z) Enlarged views of the mature rosette leaves in S–V. Scale bars: (O–R) 2 mm; (S–V) 5 mm; (W–Z) 1 mm. 360 Plant Cell Physiol. 54(3): 352–368 (2013) doi:10.1093/pcp/pcs168 ! The Author 2012. Role of FT and TSF beyond flowering arrest of lateral shoots. First, we took advantage of the inducible system for FT activity, in which the FT-T7 gene is driven by the HEAT SHOCK PROTEIN 18.2 promoter in the ft-1 background (Notaguchi et al. 2008; hereafter designated HSP:FT-T7; ft-1). HSP:FT-T7; ft-1 plants and non-transgenic ft-1 plants were grown under LD conditions until floral transition occurred and bolting began, and then the half batches of each line were heat-treated twice [on the days when they were at 2-cm-tall bolting and at 5-cm-tall bolting (Day 0), respectively]. The main and lateral shoot lengths were measured on Day 7, and were compared among heat-treated or untreated and transgenic or non-transgenic plants. The heat-treated HSP:FT-T7; ft-1 plants showed longer lateral shoots than the heat-treated ft-1 and untreated plants (Fig. 8B), while the main shoot growth was not affected by the heat shock treatment (Fig. 8A). Next, an SD to LD shift experiment was conducted. Under SD conditions, tsf-1 and ft-2; tsf-1 plants showed retardation of lateral shoot growth compared with wild-type and ft-2 plants, while the differences in flowering time among the four tested lines were much smaller than that among the lines grown under LD conditions (Fig. 3; Supplementary Table S1). Also, shift to LD conditions triggered strong induction of FT and much weaker induction of TSF in leaves (Fig. 6). If the florigen genes are involved in promotion of lateral shoot outgrowth, an SD to LD shift triggers genotype-dependent growth acceleration. Hence, the single and double mutant plants (ft-2, tsf-1 and ft-2; tsf-1) as well as wild-type (Col) plants were grown under SD conditions until Day 0, and then the half batches of each line were transferred to LD conditions. In wild-type and ft-2 plants, carrying the functional TSF gene with a high expression level in SDs and weaker induction by LDs, the lateral shoots showed modest or weak acceleration of outgrowth in response to an LD shift (Fig. 8D). In contrast, tsf-1 plants, carrying the defective TSF gene and the functional FT gene with strong induction by LDs, showed more drastic LD-induced acceleration of lateral shoot growth, and the ft-2; tsf-1 plants completely lost LD-dependent growth acceleration. Thus, these two experiments further support the idea that the florigen genes promote lateral shoot growth in Arabidopsis. Gene expression in lateral shoots of wild-type and ft plants Fig. 8 Effect of induction of florigen production on shoot growth. (A, B) Effect of FT induction in HSP:FT-T7; ft-1 plants. Lengths of the main shoot (A) and the longest lateral shoot (B) were measured on Day 7 (Day 0 was defined as the date when the main stem reached 5 cm height). The data represent the mean ± SD (n = 8 or 9). The double asterisk in B indicates P < 0.01 compared with each of the three control groups. (C, D) Effect of an SD to LD shift on lateral shoots of wild-type (Col), tsf-1, ft-2 and ft-2; tsf-1 plants. Lengths of the main shoot (C) and the longest lateral shoot (D) were measured on Day 7. The data represent the mean ± SD (n = 5 or 6). The values indicate the differences between the mean of SD-grown plants (white bars) and that of SD to LD-shifted plants (gray bars) of each line. The asterisk and the double asterisks with which the values were labeled indicate P < 0.05 and P < 0.01 when compared with SD-grown plants and SD to LD-shifted plants, respectively. The double daggers with which the white bars were labeled indicate P < 0.01 when compared with SD-grown ft-2; tsf-1 plants. To gain some insight into the effect of a compromised level of florigen, we conducted RT–qPCR analyses for selected genes in lateral shoots of wild-type (Col) and ft-2 plants grown under LD conditions. The apical regions (hereafter simply called ‘buds’) and the internodes of the lateral shoot at the axil of the lowest cauline leaves (the first lateral shoot) were sampled on the day when each plant was 2 cm tall and on Days 0, 3, 7 and 12. The collected buds contained shoot apices and flower buds at floral stage 12 or younger. The buds and internodes of the longest lateral shoots in ft plants were also sampled on Days 7 and 12 in order to distinguish the effect of FT from that of dormancy, as the first lateral shoots tended to remain in the dormant state in ft plants (Supplementary Fig. S1). DRM1 is an Arabidopsis ortholog of a dormancy-associated gene originally isolated from garden pea (Pisum sativum) and is down-regulated by decapitation (Stafstrom et al. 1998, Tatematsu et al. 2005, Aguilar-Martı́nez et al. 2007). Throughout the experimental period, we observed a higher level of DRM1 accumulation in the buds of the first lateral shoots of ft than in the buds of the longest lateral shoots of ft or in wild-type buds (Fig. 9A), suggesting that the sampled first lateral shoots in the ft plants remain in the dormant state. In internodes, DRM1 accumulation was increased over time, which may reflect the cessation of their elongation (Fig. 9B). Expression of SOC1 and AP1, two MADS-box genes which are related to the floral transition and/or flower development and are regulated by FT and TSF (Abe et al. 2005, Wigge et al. 2005, Moon et al. 2005, Yamaguchi et al. 2005, Yoo et al. 2005), was next investigated. At 2-cm-tall bolting, both SOC1 and AP1 expression in the buds was detected in wild-type and ft-2 plants Plant Cell Physiol. 54(3): 352–368 (2013) doi:10.1093/pcp/pcs168 ! The Author 2012. 361 K. Hiraoka et al. Fig. 9 Temporal expression profiles of selected genes. Buds and internodes of the lateral shoots in the axils of the lowest cauline leaves in wild-type (Col) plants (filled squares) or ft-2 plants (filled triangles) were harvested at ZT1 on the day when their main shoots reached 2 cm height and on Days 0, 3, 7 and 12. Those of the longest lateral shoot in ft-2 plants (open triangles) were also harvested on Days 7 and 12. The data which represent the means of three biological replicates were plotted as a function of time (Day after 5-cm-tall bolting). (A) Expression data of the buds. From left to right and top to bottom, DRM1, SOC1, AP1, STM, WUS, CYCD3;1, PIN1, PIN3, GA20ox1 and GA20ox2. (B) Expression data of the internodes. From left to right and top to bottom, DRM1, SOC1, STM, CYCD3;1, GA20ox1, PIN1 and PIN3. Error bars represent SDs. (Fig. 9A); the SOC1 expression level in the buds was similar in both genotypes, while the AP1 level in the buds was lower in ft-2 than in the wild type. These findings suggest that ‘floral transition’ of lateral shoots in ft-2 plants occurred by the time of 2-cm-tall bolting, but that subsequent development was delayed or somewhat altered. Consistently, AP1 expression was detected in all the lateral buds of the 1-cm-tall AP1pro: GUS transgenic (POP40) plants irrespective of ft-2 mutation (Supplementary Fig. S4). Subsequent SOC1 expression in the buds was not severely altered by ft-2 mutation. In contrast, AP1 expression in the wild-type buds showed a rapid increase to 362 reach a maximum on Day 3, while it showed an apparently slower increase toward Day 7 in the ft-2 buds. In the internodes, the SOC1 expression level in wild-type plants was much higher than that in ft-2 plants (Fig. 9B). The AP1 level in the internodes was below the limit of quantification (data not shown). Then, the expression of genes associated with meristem maintenance [STM and WUSCHEL (WUS); Lenhard et al. 2002], cell cycle [CYCLIN D3;1 (CYCD3;1); Dewitte et al. 2007], polar auxin transport (PIN1 and PIN3; Zažı́malová et al. 2010) and gibberellic acid synthesis [GIBBERELLIN 20-OXIDASE 1 (GA20ox1) and GA20ox2; Plackett et al. 2012, Rieu et al. 2008], Plant Cell Physiol. 54(3): 352–368 (2013) doi:10.1093/pcp/pcs168 ! The Author 2012. Role of FT and TSF beyond flowering all of which can potentially affect lateral shoot outgrowth, was analyzed. The expression levels of STM, CYCD3;1, PIN1 and PIN3 were not significantly affected in ft-2 plants (Fig. 9A, B). In contrast, WUS and GA20ox2 in the buds showed a somewhat similar expression profile to that of AP1 (Fig. 9A); the expression in the wild-type lateral buds showed a rapid increase, whereas that in the ft-2 buds showed a gradual increase until Day 3, followed by a steep induction. The GA20ox1 expression level in the lateral buds was not affected by ft-2 mutation, while its expression level in the ft-2 internodes was higher than that in the wild-type internodes on Day 7 (Fig. 9A, B). Thus, expression of some of the selected genes examined was affected by ft mutation in lateral buds or internodes. Discussion Regulation of lateral shoot growth by FT and TSF in Arabidopsis We have shown that ft and tsf loss-of-function mutations resulted in retardation of lateral shoot growth (Figs. 1–4) and that the recovery of FT and/or TSF expression from their deficient states led to the attenuation of the retardation (Fig. 8). The impact of the loss of the genes (i.e. the extent of retardation) in the mutants seems to reflect expression levels of the respective genes mainly in leaves (Figs. 6,7). In the experiments using LD-grown plants (Figs. 2,4), simple comparison could be difficult owing to the large differences in growth states (e.g. age, number of rosette and cauline leaves, and root density in the pot) among different genotypes to be tested. In contrast, in the experiments using plants grown under SD conditions (Fig. 3) and those carrying the inducible FT transgene (Fig. 8), and in the SD to LD and LD to SD daylength shift experiments (Figs. 5,8), plants of a similar age with similar numbers of rosette and cauline leaves could be employed so that direct comparison among different genotypes was feasible. More importantly, these experimental settings (except for that under SD conditions), which generated different activity states of the florigen genes well after the bolting of the plants, enabled us to examine the effect of florigen on lateral shoot growth independently of its effect on the floral transition of the primary shoot. Therefore, the results of these experiments led us to the unequivocal conclusion that FT and TSF regulate lateral shoot growth. It is to be noted, however, that the effect of ft single mutation on lateral shoot growth under LD conditions was not always observed (e.g. Fig. 4), and that lateral shoots of ft; tsf double mutants eventually grew out (Figs. 2,4). These findings may be because FT and TSF are not the sole crucial regulators but represent two of the important modulators of lateral shoot growth. Expression of FT and TSF in lateral shoots was detected only after they elongated, and was restricted in floral buds (Fig. 7A–H). Our recent study showed that FT is not expressed in axillary buds but is expressed in the distal part of the leaf blade of the subtending leaf (M. Niwa, M. Endo and T. Araki, unpublished observation). By using a local transient expression system, it was also demonstrated that FT protein can move into an axillary bud from the blade of the subtending leaf (M. Niwa, M. Endo and T. Araki, unpublished observation). Expression of FD, which encodes a partner of FT and TSF proteins, was clearly detected in axillary buds before starting outgrowth (M. Niwa, M. Endo and T. Araki, unpublished observation). Thus, florigen can act mainly as a non-autonomous mobile signal from leaves in the regulation of lateral shoot development as well. Interestingly, a photoperiod-independent, rather high level of expression of FT (and, to a much lesser extent, of TSF) in developing floral buds and flowers of the primary inflorescences does not seem to play a significant role in lateral shoot outgrowth (Fig. 7A–D; see also Kobayashi et al. 1999, Adrian et al. 2010). For example, lateral shoots of SD-grown ft-2 plants showed similar elongation profile to those of SD-grown wild-type plants (Fig. 3). It is likely that the utilization of inflorescence (sink)-generated florigen is much less efficient than that of leaf (source)-generated florigen. Among the genes examined in this study (Fig. 9), the expression of CYCD3;1, STM, GA20ox1, PIN1 and PIN3 was not affected by ft mutation in the apical region of the lateral shoots. In contrast, DRM1 was more strongly expressed in ft-2 plants, indicating that the lateral shoots of the mutants remained in the dormant state at least during the early stages. Consistently, the expression levels of AP1, WUS and GA20ox2 were apparently lower (less than half) in ft-2 plants. It has been well documented that the latter three genes are more strongly expressed in developing flower buds than in other tissues including internodes and leaves. AP1 is expressed in pedicels and developing flower buds from stage 1 to 10 (Mandel et al. 1992, Gustafson-Brown et al. 1994), while WUS, also involved in floral organ development, is expressed in inflorescences, with the highest expression in developing anthers (Wellmer et al. 2004, Deyhle et al. 2007). GA20ox2 is strongly expressed in inflorescences and distinctly detected in anthers and pistils of stage 12 flowers (Rieu et al. 2008, Plackett et al. 2012). Therefore, the observed difference in the expression of these three genes in the lateral buds between the wild type and ft mutant may reflect a difference in the rate of flower formation and development between the two genotypes. Actually, in addition to slower elongation rates, delay of the first flower anthesis and reduction in the number of open flowers per day were observed in lateral shoots of LD-grown ft and ft; tsf plants (Fig. 2F). Tsukaya et al. (1993) showed that elongation growth of the main shoot is regulated by the developing first flower buds. Also, Lohmann et al. (2010) recently reported that the slow motion mutation caused a slower flower formation rate and retardation of lateral shoot growth. Taking these findings together, one attractive hypothesis to be tested in the future is that the two florigen genes may regulate lateral shoot outgrowth and elongation via acceleration of flower formation. In the internodes of lateral shoots, SOC1 expression was decreased in ft plants (Fig. 9B). Melzer et al. (2008) reported that SOC1 suppressed secondary growth of the inflorescence Plant Cell Physiol. 54(3): 352–368 (2013) doi:10.1093/pcp/pcs168 ! The Author 2012. 363 K. Hiraoka et al. stem together with FUL. Involvement of FT and TSF in secondary growth and the relationship of lateral shoot development and secondary growth are interesting problems to be addressed. Differential contribution of FT and TSF to lateral shoot development under different photoperiods Differential effects of ft-2 and tsf-1 single mutations in lateral shoot outgrowth under LD and SD conditions, respectively, suggest that FT plays a more dominant role under LD conditions and TSF under SD conditions. A similar differential contribution of the two genes was previously reported in the case of floral transition (Yamaguchi et al. 2005), although the effect of the loss of TSF under SD conditions is difficult to demonstrate clearly in some experimental conditions (Michaels et al. 2005, Jang et al. 2009, this study). Therefore, our previous proposal that FT and TSF may fine-tune the timing of the floral transition and provide robustness of the process (Yamaguchi et al. 2005) seems also to be true for the regulation of lateral shoot growth by the two genes. As shown in this study, the differential contribution of the two genes is due to differential expression profiles of the respective genes mainly in leaves. Under LD conditions, FT is up-regulated in leaves much more robustly than TSF (Kobayashi et al. 1999, Yamaguchi et al. 2005), while TSF (but not FT) in leaves was gradually up-regulated in an agedependent manner under SD conditions (Fig. 6). The nature of the age-dependent cues which trigger TSF expression is currently unknown. Recently, D’Aloia et al. (2011) reported that CK applied to root was able to induce TSF expression in the shoot. Therefore, CK synthesis and/or mobilization are likely to be important components of this induction pathway. Another candidate of apparent importance is the aging pathway mediated via the miRNA156–SQUAMOSA PROMOTOR BINDING PROTEIN LIKE module (Wang et al. 2009, Yamaguchi et al. 2009). Significance of the regulation of lateral shoot outgrowth by the florigen genes Arabidopsis produces branched raceme inflorescences, with flowers being sequentially produced. The annual racemes may show high fitness, for example, if the length of the growth seasons varies from year to year (Prusinkiewicz et al. 2007). Under favorable conditions, Arabidopsis plants may exploit abundant resources (space, nutrients, water, assimilates, etc.) and hasten to produce flowers and fruits at one stretch because the best conditions will not last for a long time. In contrast, under semi-favorable conditions, the plants may keep producing flowers more slowly to ‘hedge its bets’. In this context, FT and TSF may play important roles to maximize the number of seeds under given conditions, as postulated for SFT in tomato (Krieger et al. 2010). Under LD conditions, FT plays crucial roles not only to induce floral transition synchronously (compare the standard deviations of Experiment 1 (LD) and 2 (SD) in Supplementary Table S1) but also to synchronize 364 flowering and several traits accompanying flowering such as bolting (main shoot ‘outgrowth’) and lateral shoot outgrowth and its subsequent elongation (Pouteau and Albertini 2009; Fig. 2). Therefore, FT (and TSF) may vigorously synchronize main and lateral shoot growth to guarantee fitness under favorable LD conditions. Under non-inductive SD photoperiods, their lateral shoot outgrowth was delayed, and their elongation rate and flower production were also slowed down. However, other external or internal cues also regulate lateral shoot development (Domagalska and Leyser 2011). In well-fertilized plants under SD conditions, for example, age-dependent TSF expression may support the other regulation pathways and guarantee relatively vigorous outgrowth of lateral shoots. In summary, we have shown that FT and TSF modulate the lateral shoot growth under inductive and non-inductive photoperiods, respectively, via differential induction of expression. Taken together with the findings in other plant species (Tamaki et al. 2007, Shalit et al. 2009), it is likely that the regulation of lateral shoot development is an integral part of the florigen action. To elucidate the molecular mechanism by which the florigen genes modulate lateral shoot outgrowth will be an important future challenge. In addition, differential regulation of the two florigen genes has been reported in several species (Komiya et al. 2009, Wada et al. 2010, Higuchi et al. 2011). The fine-tuning regulation of expression of the two florigen genes in Arabidopsis and other plants will be another interesting problem to be addressed. Materials and Methods Plant materials and growth conditions Arabidopsis thaliana (L.) Heynh. accession Col was mainly used as the wild type. ft-1 and ft-2 introgressed into the Col background were described in Notaguchi et al. (2008) and Imura et al. (2012), respectively. tsf-1 was described in Yamaguchi et al. (2005). ft-2, tsf-1, ft-1; soc1-2; lfy-1, ft-1; tsf-1; lfy-1 and ft-1; tsf-1; soc1-2 with or without the lfy-1 allele were newly generated in our laboratory. gFT::GUS (line AY#1) and HSP::FT-T7 (MA#2); ft-1 were described in Notaguchi et al. (2008), and gTSF::GUS (line #21-1) was described in Yamaguchi et al. (2005). POP40 was kindly provided by Dr. M.F. Yanofsky (University of California at San Diego, USA), and the POP40 line introgressed into ft-2 was generated in this study. Accessions Ler and No were also used as wild types in some experiments. ft-2 in the Ler background and ft-4, ft-5 and ft-6 in the No background were described in Kobayashi et al. (1999). ft-2; tsf-1 in the Ler background was newly generated in this study. Seeds were imbibed in water for 3 or 4 d at 4 C, and were sown on a piece of tap water-soaked Jiffy-7 peat pellet (Jiffy Products) on a pot of vermiculite. Plants were grown in growth chambers at 22 C under LD (16 h light/8 h dark) conditions with white fluorescent lights (80 mmol m–2 s–1) or under SD Plant Cell Physiol. 54(3): 352–368 (2013) doi:10.1093/pcp/pcs168 ! The Author 2012. Role of FT and TSF beyond flowering (8 h light/16 h dark) conditions with white fluorescent lights (160 mmol m–2 s–1). Measurement of shoot development In all measurements, ‘Day 0’ was defined as the day on which the main shoot of a plant reached a height of 5 cm. Shoot length was measured at a fixed time each day from when the plants started to bolt (for Figs. 2–4). The number of open flowers per day was counted at the same time. ‘The longest lateral shoot’ was defined as the lateral shoot that has been the longest during the most part of the experimental period. The elongation rate on Day X after the main shoot of a plant reached a height of 5 cm [Rday(X)] was defined as RdayðXÞ ¼ 0:5 LdayðX+1Þ LdayðX1Þ , where Lday(X) is the shoot length on Day X. ‘Open flowers’ were defined as flowers approximately at floral stages 13 and 14 (Smyth et al. 1990). Especially in ft-2; tsf-1 double mutants, flower buds with no petals (or petal-like organs) or those which never opened were observed among the early-arising buds of the inflorescences. These flower buds were not counted. To avoid double counting, one sepal of a counted flower was marked with a marker pen. Statistical analyses were performed with SPSS 19 or 20 (IBM). First, Levene’s test was used to assess the assumption of homogeneity of variances. Then, where the distribution of data met the assumptions, one-way analysis of variance followed by Tukey’s multiple comparison test was used. Otherwise, Welch’s test followed by the Games–Howell test was used. Heat shock treatment HSP::FT-T7 (MA#2); ft-1 and ft-1 plants were grown under LD conditions as described above. The height of the main shoot was measured daily at ZT7, and the plants to be treated were incubated at 40 C for 2 h (from ZT8 to ZT10) in the dark on the day when the plants reached 2 cm in height and on Day 0. GUS staining reverse-transcribed in a 20 ml reaction mixture using SuperScript III (Life Technologies; on 0.5 mg of total RNA) or Transcriptor (Roche Applied Science; on 3 mg of total RNA). After the reaction, the mixtures were diluted with 80 ml of TE buffer, and 1 ml (RT–qPCR) or 2 ml (RT–PCR) of the aliquots were used for one reaction. RT–qPCR analyses were carried out in 96-well plates with the CFX96 system (Bio-Rad Laboratories) using SYBR Green I. The primers, the reaction programs and the reaction mixture (20 ml) used for RT–qPCR are described in Supplementary Table S2. Samples were run in technical triplicates, except for the samples for the standard curve (technical duplicates). Melting curve analyses were performed from 65 to 95 C to assess the specificity of the RT–qPCR products. Quantification cycle values were converted into normalized relative quantities (NRQs) according to Hellemans et al. (2007). At4g34270 and At2g28390, the two reference genes identified by Czechowski et al. (2005), were used. Relative expression levels of each sample were calculated as the ratio of the NRQ of the sample to the NRQ of the sample from the wild-type lateral shoot buds harvested on Day 0 of the experiments, except for Fig. 6A, in which the relative expression values of FT and TSF in wild-type (Col) plants were obtained as the ratio to the level in the SD to LD shifted plants. For comparison of FT and TSF expression levels, a modified RT–PCR analysis using two restriction enzymes was performed. The primers, the reaction programs and the reaction mixture (30 ml) used in this RT–PCR analysis are listed in Supplementary Table S2. The amplified 351-bp products (a mixture of FT- and TSF-derived products) were digested by EcoT14I or PstI (FT, 243- and 108-bp fragments by PstI; TSF, 256- and 95-bp fragments by EcoT14I). Then, the digested fragments and the PCR product of ACTIN2 (as a reference gene) were electrophoresed on agarose gels and visualized by ethidium bromide staining. Supplementary data Plants were fixed for 15–30 min in 90% acetone on ice, and then incubated at 37 C for about 24 h in the dark with staining solution [0.5 mg ml–1 5-bromo-4-chloro-3-indolyl-bD-glucuronide, phosphate-buffered saline (pH 7.0; 130 mM NaCl, 7 mM Na2HPO4, 3 mM NaH2PO4), 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 0.1% (v/v) Triton X-100]. After staining, plant materials were treated with 70% ethanol to remove Chl. Then, for whole-mount observations, the samples were cleared in an 8 : 1 : 2 (w/v/v) mixture of chloral hydrate, glycerol and water. All photographs of GUS-stained samples were processed with Adobe Photoshop CS6. RT-qPCR and RT-PCR RNA was extracted using TRIzol reagent (Life Technologies) or Sepasol RNA I Super G (Nacalai Tesque), and was treated with RNase-free DNase I (Life Technologies) according to the manufacturer’s instructions. Total RNA (0.5 or 3 mg) was Supplementary data are available at PCP online. Funding This work was supported by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan [Grant-in-Aid for Scientific Research on Priority Areas (19060012, 19060016 to T.A.)]. Acknowledgments We thank Dr. M.F. Yanofsky (University of California at San Diego, USA) for POP40 seeds, Dr. T. Kohchi (Kyoto University) for apparatus, Dr. Y. Tomita of our laboratory for excellent technical assistance, and the past and present members of the Araki lab for helpful discussions and comments. Plant Cell Physiol. 54(3): 352–368 (2013) doi:10.1093/pcp/pcs168 ! The Author 2012. 365 K. Hiraoka et al. References Abe, M., Kobayashi, Y., Yamamoto, S., Daimon, Y., Yamaguchi, A., Ikeda, Y. et al. (2005) FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 309: 1052–1056. Adrian, J., Farrona, S., Reimer, J.J., Albani, M.C., Coupland, G. and Turck, F. (2010) cis-Regulatory elements and chromatin state coordinately control temporal and spatial expression of FLOWERING LOCUS T in Arabidopsis. Plant Cell 22: 1425–1440. Aguilar-Martı́nez, J.A., Poza-Carrión, C. and Cubas, P. (2007) Arabidopsis BRANCHED1 acts as an integrator of branching signals within axillary buds. Plant Cell 19: 458–472. Araki, T. (2000) Transition from vegetative to reproductive phase. Curr. Opin. Plant Biol. 4: 63–68. Bernier, G., Kinet, J-.M. and Sachs, R.M. (1981) The flowering process at the shoot apex: machromorphological events. In The Physiology of Flowering, Vol. II: pp. 21–34. Böhlenius, H., Huang, T., Charbonnel-Campaa, L., Brunner, A.M., Jansson, S., Strauss, S.H. et al. (2006) CO/FT regulatory module controls timing of flowering and seasonal growth cessation in trees. Science 312: 1040–1043. Cerdán, P.D. and Chory, J. (2003) Regulation of flowering time by light quality. Nature 423: 881–885. Chiang, G.C., Barua, D., Kramer, E.M., Amasino, R.M. and Donohue, K. (2009) Major flowering time gene, FLOWERING LOCUS C, regulates seed germination in Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 106: 11661–11666. Corbesier, L., Vincent, C., Jang, S., Fornara, F., Fan, Q., Searle, I. et al. (2007) FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 316: 1030–1033. Crawford, S., Shinohara, N., Sieberer, T., Williamson, L., George, G., Hepworth, J. et al. (2010) Strigolactones enhance competition between shoot branches by dampening auxin transport. Development 137: 2905–2913. Czechowski, T., Stitt, M., Altmann, T., Udvardi, M.K. and Scheible, W.R. (2005) Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol. 139: 5–17. D’Aloia, M., Bonhomme, D., Bouche, F., Tamseddak, K., Ormenese, S., Torti, S. et al. (2011) Cytokinin promotes flowering of Arabidopsis via transcriptional activation of the FT paralogue TSF. Plant J. 65: 972–979. Dewitte, W., Scofield, S., Alcasabas, A.A., Maughan, S.C., Menges, M., Braun, N. et al. (2007) Arabidopsis CYCD3 D-type cyclins link cell proliferation and endocycles and are rate-limiting for cytokinin responses. Proc. Natl Acad. Sci. USA 104: 14537–14542. Deyhle, F., Sarkar, A.K., Tucker, E.J. and Laux, T. (2007) WUSCHEL regulates cell differentiation during anther development. Dev. Biol. 302: 154–159. Domagalska, M.A. and Leyser, O. (2011) Signal integration in the control of shoot branching. Nat. Rev. Mol. Cell. Biol. 12: 211–221. Dorca-Fornell, C., Gregis, V., Grandi, V., Coupland, G., Colombo, L. and Kater, M.M. (2011) The Arabidopsis SOC1-like genes AGL42, AGL71 and AGL72 promote flowering in the shoot apical and axillary meristems. Plant J. 67: 1006–1017. Endo-Higashi, N. and Izawa, T. (2011) Flowering time genes Heading date 1 and Early heading date 1 together control panicle development in rice. Plant Cell Physiol. 52: 1083–1094. 366 Gustafson-Brown, C., Savidge, B. and Yanofsky, M.F. (1994) Regulation of the Arabidopsis floral homeotic gene APETALA1. Cell 76: 131–143. Hayward, A., Stirnberg, P., Beveridge, C. and Leyser, O. (2009) Interactions between auxin and strigolactone in shoot branching control. Plant Physiol. 151: 400–412. Hellemans, J., Mortier, G., De Paepe, A., Speleman, F. and Vandesompele, J. (2007) qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 8: R19. Higuchi, Y., Sage-Ono, K., Sasaki, R., Ohtsuki, N., Hoshino, A., Iida, S. et al. (2011) Constitutive expression of the GIGANTEA ortholog affects circadian rhythms and suppresses one-shot induction of flowering in Pharbitis nil, a typical short-day plant. Plant Cell Physiol. 52: 638–650. Imura, Y., Kobayashi, Y., Yamamoto, S., Furutani, M., Tasaka, M., Abe, M. et al. (2012) CRYPTIC PRECOCIOUS/MED12 is a novel flowering regulator with multiple target steps in Arabidopsis. Plant Cell Physiol. 53: 287–303. Jaeger, K.E. and Wigge, P.A. (2007) FT protein acts as a long-range signal in Arabidopsis. Curr. Biol. 17: 1050–1054. Jang, S., Torti, S. and Coupland, G. (2009) Genetic and spatial interactions between FT, TSF and SVP during the early stages of floral induction in Arabidopsis. Plant J. 60: 614–625. Kardailsky, I., Shukla, V.K., Ahn, J.H., Dagenais, N., Christensen, S.K., Nguyen, J.T. et al. (1999) Activation tagging of the floral inducer FT. Science 286: 1962–1965. Kaufmann, K., Wellmer, F., Muiño, J.M., Ferrier, T., Wuest, S.E., Kumar, V. et al. (2010) Orchestration of floral initiation by APETALA1. Science 328: 85–89. Kinoshita, T., Ono, N., Hayashi, Y., Morimoto, S., Nakamura, S., Soda, M. et al. (2011) FLOWERING LOCUS T regulates stomatal opening. Curr. Biol. 21: 1232–1238. Kobayashi, Y., Kaya, H., Goto, K., Iwabuchi, M. and Araki, T. (1999) A pair of related genes with antagonistic roles in mediating flowering signals. Science 286: 1960–1962. Komiya, R., Yokoi, S. and Shimamoto, K. (2009) A gene network for long-day flowering activates RFT1 encoding a mobile flowering signal in rice. Development 136: 3443–3450. Krieger, U., Lippman, Z.B. and Zamir, D. (2010) The flowering gene SINGLE FLOWER TRUSS drives heterosis for yield in tomato. Nat. Genet. 42: 459–463. Lee, J. and Lee, I. (2010) Regulation and function of SOC1, a flowering pathway integrator. J. Exp. Bot. 61: 2247–2254. Lenhard, M., Jürgens, G. and Laux, T. (2002) The WUSCHEL and SHOOTMERISTEMLESS genes fulfil complementary roles in Arabidopsis shoot meristem regulation. Development 129: 3195–3206. Levy, Y.Y. and Dean, C. (1998) The transition to flowering. Plant Cell 10: 1973–1989. Liu, C., Xi, W., Shen, L., Tan, C. and Yu, H. (2009) Regulation of floral patterning by flowering time genes. Dev. Cell 16: 711–722. Lohmann, D., Stacey, N., Breuninger, H., Jikumaru, Y., Müller, D., Sicard, A. et al. (2010) SLOW MOTION is required for within-plant auxin homeostasis and normal timing of lateral organ initiation at the shoot meristem in Arabidopsis. Plant Cell 22: 335–348. Mandel, M.A., Gustafson-Brown, C., Savidge, B. and Yanofsky, M.F. (1992) Molecular characterization of the Arabidopsis floral homeotic gene APETALA1. Nature 360: 273–277. Plant Cell Physiol. 54(3): 352–368 (2013) doi:10.1093/pcp/pcs168 ! The Author 2012. Role of FT and TSF beyond flowering Mathieu, J., Warthmann, N., Küttner, F. and Schmid, M. (2007) Export of FT protein from phloem companion cells is sufficient for floral induction in Arabidopsis. Curr. Biol. 17: 1055–1060. Melzer, S., Lens, F., Gennen, J., Vanneste, S., Rohde, A. and Beeckman, T. (2008) Flowering-time genes modulate meristem determinacy and growth form in Arabidopsis thaliana. Nat. Genet. 40: 1489–1492. Michaels, S.D., Himelblau, E., Kim, S.Y., Schomburg, F.M. and Amasino, R.M. (2005) Integration of flowering signals in winterannual Arabidopsis. Plant Physiol. 137: 149–156. Minakuchi, K., Kameoka, H., Yasuno, N., Umehara, M., Luo, L., Kobayashi, K. et al. (2010) FINE CULM1 (FC1) works downstream of strigolactones to inhibit the outgrowth of axillary buds in rice. Plant Cell Physiol. 51: 1127–1135. Moon, J., Lee, H., Kim, M. and Lee, I. (2005) Analysis of flowering pathway integrators in Arabidopsis. Plant Cell Physiol. 46: 292–299. Moyroud, E., Kusters, E., Monniaux, M., Koes, R. and Parcy, F. (2010) LEAFY blossoms. Trends Plant Sci. 15: 346–352. Müller, D. and Leyser, O. (2011) Auxin, cytokinin and the control of shoot branching. Ann. Bot. 107: 1203–1212. Navarro, C., Abelenda, J.A., Cruz-Oró, E., Cuéllar, C.A., Tamaki, S., Silva, J. et al. (2011) Control of flowering and storage organ formation in potato by FLOWERING LOCUS T. Nature 478: 119–122. Notaguchi, M., Abe, M., Kimura, T., Daimon, Y., Kobayashi, T., Yamaguchi, A. et al. (2008) Long-distance, graft-transmissible action of Arabidopsis FLOWERING LOCUS T protein to promote flowering. Plant Cell Physiol. 49: 1645–1658. Ongaro, V., Bainbridge, K., Williamson, L. and Leyser, O. (2008) Interactions between axillary branches of Arabidopsis. Mol. Plant 1: 388–400. Osnato, M., Castillejo, C., Matı́as-Hernández, L. and Pelaz, S. (2012) TEMPRANILLO genes link photoperiod and gibberellin pathways to control flowering in Arabidopsis. Nat. Commun. 3: 808. Plackett, A.R., Powers, S.J., Fernandez-Garcia, N., Urbanova, T., Takebayashi, Y., Seo, M. et al. (2012) Analysis of the developmental roles of the Arabidopsis gibberellin 20-oxidases demonstrates that GA20ox1, -2, and -3 are the dominant paralogs. Plant Cell 24: 941–960. Pouteau, S. and Albertini, C. (2009) The significance of bolting and floral transitions as indicators of reproductive phase change in Arabidopsis. J. Exp. Bot. 60: 3367–3377. Prusinkiewicz, P., Crawford, S., Smith, R.S., Ljung, K., Bennett, T., Ongaro, V. et al. (2009) Control of bud activation by an auxin transport switch. Proc. Natl Acad. Sci. USA 106: 17431–17436. Prusinkiewicz, P., Erasmus, Y., Lane, B., Harder, L.D. and Coen, E. (2007) Evolution and development of inflorescence architectures. Science 316: 1452–1456. Purwestri, Y.A., Ogaki, Y., Tamaki, S., Tsuji, H. and Shimamoto, K. (2009) The 14-3-3 protein GF14c acts as a negative regulator of flowering in rice by interacting with the florigen Hd3a. Plant Cell Physiol. 50: 429–438. Reeves, P.H. and Coupland, G. (2001) Analysis of flowering time control in Arabidopsis by comparison of double and triple mutants. Plant Physiol. 126: 1085–1091. Rieu, I., Ruiz-Rivero, O., Fernandez-Garcia, N., Griffiths, J., Powers, S.J., Gong, F. et al. (2008) The gibberellin biosynthetic genes AtGA20ox1 and AtGA20ox2 act, partially redundantly, to promote growth and development throughout the Arabidopsis life cycle. Plant J. 53: 488–504. Ruiz-Garcı́a, L., Madueño, F., Wilkinson, M., Haughn, G., Salinas, J. and Martı́nez-Zapater, J.M. (1997) Different roles of flowering-time genes in the activation of floral initiation genes in Arabidopsis. Plant Cell 9: 1921–1934. Shalit, A., Rozman, A., Goldshmidt, A., Alvarez, J.P., Bowman, J.L., Eshed, Y. et al. (2009) The flowering hormone florigen functions as a general systemic regulator of growth and termination. Proc. Natl Acad. Sci. USA 106: 8392–8397. Shimizu, S. and Mori, H. (1998) Analysis of cycles of dormancy and growth in pea axillary buds based on mRNA accumulation patterns of cell cycle-related genes. Plant Cell Physiol. 39: 255–262. Smith, H.M., Ung, N., Lal, S. and Courtier, J. (2011) Specification of reproductive meristems requires the combined function of SHOOT MERISTEMLESS and floral integrators FLOWERING LOCUS T and FD during Arabidopsis inflorescence development. J. Exp. Bot. 62: 583–593. Smyth, D.R., Bowman, J.L. and Meyerowitz, E.M. (1990) Early flower development in Arabidopsis. Plant Cell 2: 755–767. Srikanth, A. and Schmid, M. (2011) Regulation of flowering time: all roads lead to Rome. Cell Mol. Life Sci. 68: 2013–2037. Stafstrom, J.P., Ripley, B.D., Devitt, M.L. and Drake, B. (1998) Dormancy-associated gene expression in pea axillary buds. Cloning and expression of PsDRM1 and PsDRM2. Planta 205: 547–552. Suárez-López, P., Wheatley, K., Robson, F., Onouchi, H., Valverde, F. and Coupland, G. (2001) CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature 410: 1116–1120. Takada, S. and Goto, K. (2003) TERMINAL FLOWER2, an Arabidopsis homolog of HETEROCHROMATIN PROTEIN1, counteracts the activation of FLOWERING LOCUS T by CONSTANS in the vascular tissues of leaves to regulate flowering time. Plant Cell 15: 2856–2865. Tamaki, S., Matsuo, S., Wong, H.L., Yokoi, S. and Shimamoto, K. (2007) Hd3a protein is a mobile flowering signal in rice. Science 316: 1033–1036. Tanaka, M., Takei, K., Kojima, M., Sakakibara, H. and Mori, H. (2006) Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance. Plant J. 45: 1028–1036. Taoka, K., Ohki, I., Tsuji, H., Furuita, K., Hayashi, K., Yanase, T. et al. (2011) 14-3-3 proteins act as intracellular receptors for rice Hd3a florigen. Nature 476: 332–335. Tatematsu, K., Ward, S., Leyser, O., Kamiya, Y. and Nambara, E. (2005) Identification of cis-elements that regulate gene expression during initiation of axillary bud outgrowth in Arabidopsis. Plant Physiol. 138: 757–766. Torti, S., Fornara, F., Vincent, C., Andrés, F., Nordström, K., Göbel, U. et al. (2012) Analysis of the Arabidopsis shoot meristem transcriptome during floral transition identifies distinct regulatory patterns and a leucine-rich repeat protein that promotes flowering. Plant Cell 24: 444–462. Tsukaya, H., Naito, S., Rédei, G.P. and Komeda, Y. (1993) A new class of mutations in Arabidopsis thaliana, acaulis1, affecting the development of both inflorescences and leaves. Development 118: 751–764. Wada, K.C., Yamada, M., Shiraya, T. and Takeno, K. (2010) Salicylic acid and the flowering gene FLOWERING LOCUS T homolog are involved in poor-nutrition stress-induced flowering of Pharbitis nil. J. Plant Physiol. 167: 447–452. Plant Cell Physiol. 54(3): 352–368 (2013) doi:10.1093/pcp/pcs168 ! The Author 2012. 367 K. Hiraoka et al. Wang, J.W., Czech, B. and Weigel, D. (2009) miR156-regulated SPL transcription factors define an endogenous flowering pathway in Arabidopsis thaliana. Cell 138: 738–749. Wellmer, F., Riechmann, J.L., Alves-Ferreira, M. and Meyerowitz, E.M. (2004) Genome-wide analysis of spatial gene expression in Arabidopsis flowers. Plant Cell 16: 1314–1326. Wigge, P.A., Kim, M.C., Jaeger, K.E., Busch, W., Schmid, M., Lohmann, J.U. et al. (2005) Integration of spatial and temporal information during floral induction in Arabidopsis. Science 309: 1056–1059. Willmann, M.R. and Poethig, R.S. (2011) The effect of the floral repressor FLC on the timing and progression of vegetative phase change in Arabidopsis. Development 138: 677–685. Yamaguchi, A. and Abe, M. (2012) Regulation of reproductive development by non-coding RNA in Arabidopsis: to flower or not to flower. J. Plant Res. 125: 693–704. 368 Yamaguchi, A., Kobayashi, Y., Goto, K., Abe, M. and Araki, T. (2005) TWIN SISTER OF FT (TSF) acts as a floral pathway integrator redundantly with FT. Plant Cell Physiol. 46: 1175–1189. Yamaguchi, A., Wu, M.F., Yang, L., Wu, G., Poethig, R.S. and Wagner, D. (2009) The microRNA-regulated SBP-Box transcription factor SPL3 is a direct upstream activator of LEAFY, FRUITFULL, and APETALA1. Dev. Cell 17: 268–278. Yanovsky, M.J. and Kay, S.A. (2002) Molecular basis of seasonal time measurement in Arabidopsis. Nature 419: 308–312. Yoo, S.K., Chung, K.S., Kim, J., Lee, J.H., Hong, S.M., Yoo, S.J. et al. (2005) CONSTANS activates SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 through FLOWERING LOCUS T to promote flowering in Arabidopsis. Plant Physiol. 139: 770–778. Zažı́malová, E., Murphy, A.S., Yang, H., Hoyerová, K. and Hošek, P. (2010) Auxin transporters—why so many? Cold Spring Harb. Perspect. Biol. 2: a001552. Plant Cell Physiol. 54(3): 352–368 (2013) doi:10.1093/pcp/pcs168 ! The Author 2012.