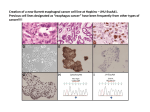
* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Full-Text PDF
Neuropharmacology wikipedia , lookup
Psychopharmacology wikipedia , lookup
Cell encapsulation wikipedia , lookup
Discovery and development of proton pump inhibitors wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Discovery and development of neuraminidase inhibitors wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Prescription costs wikipedia , lookup
Development of analogs of thalidomide wikipedia , lookup
Drug interaction wikipedia , lookup
Drug design wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Theralizumab wikipedia , lookup
Drug discovery wikipedia , lookup
International Journal of Molecular Sciences Review Recent Advances in Nanoparticle-Mediated Delivery of Anti-Inflammatory Phytocompounds Raffaele Conte 1,† , Valentina Marturano 2,3,† , Gianfranco Peluso 1 , Anna Calarco 1, * and Pierfrancesco Cerruti 2 1 2 3 * † Institute of Agro-Environmental and Forest Biology (IBAF-CNR), Via Pietro Castellino 111, 80131 Napoli, Italy; [email protected] (R.C.); [email protected] (G.P.) Institute for Polymers, Composites, and Biomaterials (IPCB-CNR), Via Campi Flegrei 34, 80078 Pozzuoli (NA), Italy; [email protected] (V.M.); [email protected] (P.C.) Department of Chemical Sciences, University of Naples “Federico II”, Via Cynthia 4, 80125 Napoli, Italy Correspondence: [email protected]; Tel.: +39-081-6132569 These authors contributed equally to this work. Academic Editors: Paula Andrade and Patrícia Valentão Received: 1 February 2017; Accepted: 23 March 2017; Published: 28 March 2017 Abstract: Phytocompounds have been used in medicine for decades owing to their potential in anti-inflammatory applications. However, major difficulties in achieving sustained delivery of phyto-based drugs are related to their low solubility and cell penetration, and high instability. To overcome these disadvantages, nanosized delivery technologies are currently in use for sustained and enhanced delivery of phyto-derived bioactive compounds in the pharmaceutical sector. This review focuses on the recent advances in nanocarrier-mediated drug delivery of bioactive molecules of plant origin in the field of anti-inflammatory research. In particular, special attention is paid to the relationship between structure and properties of the nanocarrier and phytodrug release behavior. Keywords: inflammation; phytochemicals; nanosized delivery systems; polyphenols; carbohydrates; cannabinoids; terpenoids; essential oils; nanoparticles; nanocapsules 1. Introduction Inflammation represents the physiological response of the body to tissue injury (e.g., stress, irritants, and radiations), infections (microbial and viral) or genetic changes and may be distinguished into two phases: acute and chronic. The acute is the early nonspecific phase characterized by local vasodilatation, increased capillary permeability, accumulation of fluid and blood proteins into the interstitial spaces, migration of neutrophils out of the capillaries, and release of inflammatory mediators (e.g., cytokines, lymphokines, and histamine) [1–6]. Clinically, acute inflammation is characterized by five cardinal signs: rubor (redness), calor (increased heat), tumor (swelling), dolor (pain), and functio laesa (loss of function). Under normal conditions, neutrophils undergo apoptosis after performing their action at the inflamed site and macrophages ingest apoptotic neutrophils. Clearance of apoptotic neutrophils prompts a switch from a pro- to an anti-inflammatory macrophage phenotype, which enable macrophage egress favoring tissue repair and regain of physiological function (Figure 1A) [7–12]. Int. J. Mol. Sci. 2017, 18, 709; doi:10.3390/ijms18040709 www.mdpi.com/journal/ijms Int. J. Mol. Sci. 2017, 18, 709 2 of 23 Int. J. Mol. Sci. 2017, 18, 709 2 of 23 Figure 1.1.Schematic representation of the main characteristics of: acute (A) of: and chronic (B) inflammation. Figure Schematic representation of the main characteristics acute (A) and chronic (B) inflammation. However, if the condition causing the damage is not resolved, the inflammatory process evolves toward if subacute/chronic inflammation characterized immunopathological changes such However, the condition causing the damage is not by resolved, the inflammatory process as infiltration inflammatory cells, overexpression of pro-inflammatory genes, dysregulation of evolves towardofsubacute/chronic inflammation characterized by immunopathological changes such cellular signaling and loss of barrier function. The chronic state of inflammation has important roles as infiltration of inflammatory cells, overexpression of pro-inflammatory genes, dysregulation of in the onset of various diseases, including cardiovascular neurodegenerative diseases, cellular signaling and loss of barrier function. The chronic disease, state of inflammation has important diabetes, cancer, obesity, asthma, as well as classic inflammatory diseases (e.g., arthritis and roles in the onset of various diseases, including cardiovascular disease, neurodegenerative diseases, periodontal diseases) (Figure 1B) [13–23]. In the last decade, a new family of clinical disorders, diabetes, cancer, obesity, asthma, as well as classic inflammatory diseases (e.g., arthritis and periodontal namely auto-inflammatory diseases, Auto-inflammatory disorders are systemic a group diseases)systemic (Figure 1B) [13–23]. In the last decade,emerged. a new family of clinical disorders, namely of heterogeneous disorders characterized by apparently inexplicable recurrence of local auto-inflammatory diseases, emerged. Auto-inflammatory disorders are a group of heterogeneous inflammation, with an increased production of inflammatory cytokines that can occur without disorders characterized by apparently inexplicable recurrence of local inflammation, with an increased detectable autoantibodies auto-reactive T cells. thesedetectable diseases are caused by overproduction production of inflammatoryorcytokines that can occur All without autoantibodies or auto-reactive of pro-inflammatory cytokines, such as interleukin (IL)-1β and tumor necrosis factor T cells. All these diseases are caused by overproduction of pro-inflammatory cytokines,(TNF)-α, such as abnormal sensing in inflammatory bowel disease), and tissue micro-damage leading interleukinmicrobial (IL)-1β and tumor(as necrosis factor (TNF)-α, abnormal microbial sensing (as in inflammatory to a pathological delay in the turning off of inflammatory responses [24–26]. bowel disease), and tissue micro-damage leading to a pathological delay in the turning off of Several evidences inflammatory responsessuggest [24–26].that the regulation of inflammation is tightly controlled by an array of epigenetic mechanisms and that cannot confinedofto a simple model in which the expression ofof a Several evidences suggest the be regulation inflammation is tightly controlled by an array distinct set of genes depends exclusively on a single or a defined set of transcription factors [27,28]. epigenetic mechanisms and cannot be confined to a simple model in which the expression of a distinct or inhibition mediators using synthetic antiset ofSuppression genes depends exclusivelyofoninflammatory/pro-inflammatory a single or a defined set of transcription factors [27,28]. inflammatory compounds (e.g., of steroidal and nonsteroidal) is one ofmediators the majorusing routessynthetic for the Suppression or inhibition inflammatory/pro-inflammatory treatment of inflammatory disorders. However, the use of synthetic anti-inflammatory molecules is anti-inflammatory compounds (e.g., steroidal and nonsteroidal) is one of the major routes for the associated with some common side effects including gastric irritation, ulceration, bleeding, renal treatment of inflammatory disorders. However, the use of synthetic anti-inflammatory molecules failure, hepatic failure, headache, hemolytic anemia, asthma exacerbation, skin rashes, angioedema, is associated with some common side effects including gastric irritation, ulceration, bleeding, and recent years, phytochemicals derived from plants have gained increased renalpruritus failure, [29,30]. hepaticInfailure, headache, hemolytic anemia, asthma exacerbation, skin rashes, attention dueand to their safe [29,30]. toxicological profiles to allopathic the treatment of angioedema, pruritus In recent years,compared phytochemicals deriveddrugs from in plants have gained inflammatory disease [31–34], and their protective action has been demonstrated in obesityincreased attention due to their safe toxicological profiles compared to allopathic drugs in the treatment mediated inflammation [35,36],and inflammatory bowel disease [37–40], Alzheimer disease [41,42], of inflammatory disease [31–34], their protective action has been demonstrated in obesity-mediated diabetes [43], skin inflammation [44,45]. However, despite Alzheimer promising disease biological activities of plant inflammation [35,36], inflammatory bowel disease [37–40], [41,42], diabetes [43], raw extracts, poor solubility, poor stability, short biological half-life, and rapid elimination hinder skin inflammation [44,45]. However, despite promising biological activities of plant raw extracts, their clinical application. Moreover, their large molecular size negatively affects passive absorption, Int. J. Mol. Sci. 2017, 18, 709 3 of 23 poor solubility, poor stability, short biological half-life, and rapid elimination hinder their clinical application. Moreover, their large molecular size negatively affects passive absorption, and their pharmacokinetics is further modified by the highly acidic gastric pH and pre-systemic metabolism [46]. These aspects, together with the individual variation of extracts constituents, led research interest towards the synthesis of systems able to effectively deliver these substances. 2. Nanosized Drug Delivery Systems Nanotechnology is already employed in traditional drug delivery and tissue engineering [47], as it offers the possibility to develop devices particularly adapted to improve the therapeutic efficacy of natural bioactive molecules [48]. Indeed, nanocarriers are alternatives for traditional formulation approaches, enabling to increase drug bioavailability, permit a site-specific targeted delivery, as well as reducing toxic side effects [49–51]. Since the 1970s, when Nobel Prize winner Christian de Duve described the structure and properties of lysosomes in biological tissues, drug administration protocols have significantly evolved due to the introduction of nanosized drug delivery systems [52]. The latter can be defined as ultra-dispersed solid organic or inorganic structures displaying a sub-micrometer size, typically comprised between 10 and 100 nm. The upper limit is dictated by the vector’s ability to pass cellular interstices, while the lower limit is fixed by the threshold for first-pass elimination by kidneys. Moreover, such dimensions permit a good biodistribution of long-circulating nanocarriers [53]. If compared to traditional formulations, the use of nanovectors for drug delivery offers many advantages: more efficient delivery of water insoluble drugs at high dose, protection for the drug from hostile environments (e.g., acidic pH in the digestive tract), and targeted and controlled delivery to achieve precise administration to a specific tissue over a determined period of time [54]. Various materials and structures have been employed as nanocarriers for either passive or active targeting. Metal particles, such as iron oxide or gold nanosized assemblies, can be surface modified to act as drug carriers [55,56]. However, organic materials are more versatile, and their properties and performances can be finely tuned by tailoring their chemical composition, size, shape, structures morphology, and surface properties [57], as depicted in Figure 2. Lipid-based particles, such as liposomes, or cubosomes, are supramolecular assemblies of amphiphilic molecules, in which hydrophobic drugs can be encapsulated [58,59]. Another class of natural carriers employed in drug delivery is cyclodextrins (CD), cyclic oligosaccharides characterized by a hydrophilic outer surface, and able to load guest molecules in their lipophilic inner cavity via non-covalent inclusion interactions. As well as other nanocarriers, the use of CD can increase drug solubility, bioavailability, safety, and stability of drug formulations [60]. Polymers provide even more versatility in nanocarriers engineering, being nanoparticles (NP), nanocapsules (NC), dendrimers and copolymer micelles among the numerous examples of polymer-based assemblies that serve as a reservoir for the loaded drug [61]. In nanoparticles and nanocapsules, drugs can be encapsulated in or conjugated to polymer chains. Dendrimers are made up of several branched polymer chains stemming from a central core, in which the active principle can be conjugated or complexed. Polymer micelles result from the self-assembly in water of amphiphilic copolymers into a core–shell structure. The hydrophobic core can be act as a reservoir of hydrophobic drugs while the hydrophilic corona provides water solubility and colloidal stability [62]. Tailored engineering and design of polymeric carriers allow precise and controlled administration of the cargo drug. A passive delivery occurs when the loaded drug is released by diffusion or erosion of the nanovector, an active delivery can instead be achieved employing stimuli-responsive materials as drug carriers. In stimuli-responsive (or smart) materials, a variation in structure or morphology is triggered by an external stimulus, such as pH variations, contact with concentrated ionic solution or enzymes, exposition to light, ultrasound, electric field, magnetic field or heating [63]. Int. J. Mol. Sci. 2017, 18, 709 Int. J. Mol. Sci. 2017, 18, 709 4 of 23 4 of 23 Figure Figure 2. 2. Examples Examples of of organic organic material-based material-based nanosized nanosized drug drug delivery delivery systems. systems. In this frame, the surface properties of the nanocarrier determine the interaction with body In this frame, the surface properties of the nanocarrier determine the interaction with body environment to a great extent [64]. For example, nanovectors with negative or positive surface environment to a great extent [64]. For example, nanovectors with negative or positive surface charges have increased reticuloendothelial clearance. Steric stabilization with biological or synthetic charges have increased reticuloendothelial clearance. Steric stabilization with biological or synthetic macromolecules, such as opsonin or polyethylene glycol (PEG), prolongs circulation times and macromolecules, such as opsonin or polyethylene glycol (PEG), prolongs circulation times and prevents prevents nanoparticle loss [65]. Targeting strategies are aimed to overcome side effects and nanoparticle loss [65]. Targeting strategies are aimed to overcome side effects and minimize systemic minimize systemic drug administration. These are classified into passive and active targeting [66]. drug administration. These are classified into passive and active targeting [66]. Passive targeting is Passive targeting is achieved by changing the physiochemical characteristics, pH or hydrophobicity achieved by changing the physiochemical characteristics, pH or hydrophobicity of NP and utilizes the of NP and utilizes the “enhanced permeability and retention effect” of inflamed blood vessels. “enhanced permeability and retention effect” of inflamed blood vessels. Active targeting, differently, Active targeting, differently, uses biomarkers (e.g., mutant genes, RNAs, proteins, lipids, uses biomarkers (e.g., mutant genes, RNAs, proteins, lipids, carbohydrates, and small metabolite carbohydrates, and small metabolite molecules) to reach the target sites [67]. The addition of a molecules) to reach the target sites [67]. The addition of a targeting moiety onto the surface of NP targeting moiety onto the surface of NP increases selective cellular binding and internalization increases selective cellular binding and internalization through receptor-mediated endocytosis [53]. through receptor-mediated endocytosis [53]. In this regard, key properties of nanoparticles (NP) In this regard, key properties of nanoparticles (NP) utilized as plant-derived anti-inflammatory delivery utilized as plant-derived anti-inflammatory delivery systems greatly depend on size, surface systems greatly depend on size, surface characteristics, and targeting strategies. characteristics, and targeting strategies. Even though the use of nanocarriers as drug-delivery systems offers many advantages, Even though the use of nanocarriers as drug-delivery systems offers many advantages, some some drawbacks need to be addressed, e.g., instability during blood circulation, low renal clearance, drawbacks need to be addressed, e.g., instability during blood circulation, low renal clearance, limited accumulation in specific tissues, and low uptake by target cells. Moreover, case by case limited accumulation in specific tissues, and low uptake by target cells. Moreover, case by case evaluation of the interactions between nanocarriers and biological systems is of key importance to evaluation of the interactions between nanocarriers and biological systems is of key importance to assess the reliability of the delivery systems. The successful translation of nano-formulations to the assess the reliability of the delivery systems. The successful translation of nano-formulations to the clinic involves careful assessment of their safety profiles, which, among other end-points, includes the clinic involves careful assessment of their safety profiles, which, among other end-points, includes evaluation of immunotoxicity [68]. Moreover, since physico-chemical properties of nanoparticles, the evaluation of immunotoxicity [68]. Moreover, since physico-chemical properties of such as surface charge and size, modulate uptake and interactions with cells, control over surface nanoparticles, such as surface charge and size, modulate uptake and interactions with cells, control modification and biodegradation of nanovectors contributes to minimize potential health risks that are over surface modification and biodegradation of nanovectors contributes to minimize potential associated with occupational exposure to such materials [69]. The main challenges associated with health risks that are associated with occupational exposure to such materials [69]. The main nano-toxicity evaluations are related to the development of more accurate and fully-characterized challenges associated with nano-toxicity evaluations are related to the development of more reference materials and methods. In addition, there is the need to develop a paradigm for predictive accurate and fully-characterized reference materials and methods. In addition, there is the need to toxicological testing of nanomaterials, taking into consideration the physico-chemical properties of the develop a paradigm for predictive toxicological testing of nanomaterials, taking into consideration material that lead to molecular or cellular injury, with the aim to triage novel nanomaterials for further the physico-chemical properties of the material that lead to molecular or cellular injury, with the in vivo testing [70]. aim to triage novel nanomaterials for further in vivo testing [70]. This review provides a focus on recent advances in the preparation and characterization This review provides a focus on recent advances in the preparation and characterization of of nanosized vectors able to release phytochemicals endowed with anti-inflammatory activity. nanosized vectors able to release phytochemicals endowed with anti-inflammatory activity. Different delivery technologies will be discussed, according to chemical structure and natural source Different delivery technologies will be discussed, according to chemical structure and natural source of the bioactive molecules to be released. In particular, organic nanovectors loaded with Int. J. Mol. Sci. 2017, 18, 709 5 of 23 Int. J. Mol. Sci. 2017, 18, 709 5 of 23 of the bioactive molecules to be released. In particular, organic nanovectors loaded with polyphenols, phytocannabinoids, phytosterols, carbohydrates, essential oils, and terpenoids will be reviewed polyphenols, phytocannabinoids, phytosterols, carbohydrates, essential oils, and terpenoids will be (Figure 3). For each 3). phytochemical class, a focus will be given togiven the relationship between structure reviewed (Figure For each phytochemical class, a focus will be to the relationship between andstructure properties of nanocarriers and phytodrugs release behavior. and properties of nanocarriers and phytodrugs release behavior. Figure 3. Nanoparticle-mediated delivery of different classes of anti-inflammatory natural compounds. Figure 3. Nanoparticle-mediated delivery of different classes of anti-inflammatory natural compounds. 3. Anti-Inflammatory Phytoconstituents and Their Nanoparticle-Mediated Delivery 3. Anti-Inflammatory Phytoconstituents and Their Nanoparticle-Mediated Delivery 3.1. Polyphenols 3.1. Polyphenols Polyphenols (PPH) are a large family of ubiquitous and varied molecules present as secondary Polyphenols (PPH) arevascular a large plants. familyThese of ubiquitous and varied molecules present as secondary metabolites in numerous natural compounds range from simple molecules to complex structures that have in common the presence of benzene rings bearing one or several metabolites in numerous vascular plants. These natural compounds range from simple molecules hydroxylstructures functions. that In the plant, these active principlesofplay an important role in to complex have in common the presence benzene rings bearing onegrowth, or several reproduction, resistance to pathogens, predators and play diseases [71]. Nonetheless, PPH are potent hydroxyl functions. In the plant, these active principles an important role in growth, reproduction, effectors biologic processes associated with [71]. the pathogenesis human thanks tooftheir resistance to of pathogens, predators and diseases Nonetheless,ofPPH are diseases potent effectors biologic ability to interact with proteins, enzymes and membrane receptors, modulating the resulting processes associated with the pathogenesis of human diseases thanks to their ability to interact with activity [72]. Among their properties, the strong free radical scavenging action is probably the most proteins, enzymes and membrane receptors, modulating the resulting activity [72]. Among their studied, and is involved in the anti-inflammatory action of these molecules. In particular, quercetin properties, the strong free radical scavenging action is probably the most studied, and is involved (QC), resveratrol (RE) and tannins are recognized as pain killers [73]. PPH are able to improve in the anti-inflammatory of these particular, quercetin (QC), resveratrol health by strengtheningaction the response of molecules. the immuneIn system towards chronic diseases. However, (RE) andtheir tannins are recognized painmetabolism killers [73]. PPH are able to improve by strengthening efficacy depends on as their and bioavailability, defined ashealth the amount of the the administrated response of the system towards chronic diseases.InHowever, their efficacy depends drugimmune that is able to reach the systemic circulation. general, PPH have a relatively on their metabolism and as the amount of the administrated druglow that is low bioavailability due bioavailability, to both intrinsicdefined factors (chemical structure, molecular weight and and extrinsic factors In (low stabilityPPH in the gastrointestinal environment, extensive ablehydrosolubility) to reach the systemic circulation. general, have a relatively low bioavailability due to II metabolism and rapid elimination) [74]. Consequently, clinical applications of and PPHextrinsic are bothphase intrinsic factors (chemical structure, molecular weight and low hydrosolubility) limited. avoid these drawbacks, nanodelivery systems able to maintain theIIstructural integrity factors (lowTostability in the gastrointestinal environment, extensive phase metabolism andof rapid these bioactive molecules have been developed [75]. For example, nanosized vectors encapsulating elimination) [74]. Consequently, clinical applications of PPH are limited. To avoid these drawbacks, QC, a semi-lipophilic flavonol ubiquitous in plants, have been developed by Li et al. through the nanodelivery systems able to maintain the structural integrity of these bioactive molecules have synthesis of solid lipid nanoparticles (SLN) of 155 nm of size composed of soya lecithin, Tween-80 been developed [75]. For example, nanosized vectors encapsulating QC, a semi-lipophilic flavonol and polyethylene glycol (PEG), with a QC encapsulation efficiency of 91% [76]. These NP were able ubiquitous in plants, have been developed by Li et al. through the synthesis of solid lipid nanoparticles to increase the relative oral bioavailability of quercetin by 5.7-fold as compared to the free form. (SLN) of 155 nm nanocapsules of size composed soyabeen lecithin, Tween-80 and polyethylene (PEG), Lipid-coated (NC)ofhave reported by Barras et al., with a glycol solubility 100 with timesa QC encapsulation 91% [76]. These NPfor were able to increase theand relative bioavailability higher withefficiency respect toof free quercetin, stable more than ten weeks, with oral no degradation of quercetin by 5.7-fold as compared to the free form. Lipid-coated nanocapsules (NC) have been product being detected [77]. Pool et al. produced quercetin-loaded polylactic-co-glycolide (PLGA) reported by Barras et al., with a solubility 100 times higher with respect to free quercetin, stable for NC with a more potent antioxidant action against peroxyl radical-induced lipid peroxidation, resulting in weeks, a moreand effective anti-inflammatory therapybeing [78]. detected Similarly, [77]. Wu et al. et synthesized more than ten with no degradation product Pool al. produced quercetin-loaded polylactic-co-glycolide (PLGA) NC with a more potent antioxidant action against peroxyl radical-induced lipid peroxidation, resulting in a more effective anti-inflammatory therapy [78]. Int. J. Mol. Sci. 2017, 18, 709 6 of 23 Similarly, Wu et al. synthesized quercetin-loaded Eudragit-polyvinyl alcohol NP with particle size of 85 nm, good polydispersity, drug loading of around 99% and enhanced antioxidant activity [79]. Finally, Chakraborty et al. examined the potency of orally administered quercetin-PLGA NP in a rat model [80]. Nanoparticles were administered prior to alcohol induced gastric ulcer in the protection against oxidative damages, demonstrating that QC-PLGA NP prevented 90% of this inflammation as compared to the 20% of the free quercetin. Resveratrol, chemically known as 3,5,4-trihydroxystilbene, is a naturally occurring polyphenol produced by a wide variety of plants in response to injury, UV irradiation, ozone exposure and fungal attack [81]. Nanovectors delivering RE have been reported by Singh and Pai, that described a sustained release of trans-resveratrol from orally administered PLGA NP (drug encapsulation efficiency more than 78%, with a particle size of about 170 nm) [82]. The same authors encapsulated RE in Eudragit RL 100 NP with a drug incorporation efficiency of 84% and average size of 180 nm. In vivo studies in a rat model showed prolonged plasma levels up to 16 h, in comparison with free drug being cleared within 6 h [83]. Zu et al. developed carboxymethyl chitosan NP as carrier for resveratrol [84]. These nanoparticles (155 nm-sized, with an encapsulation efficiency of 44%) improved the solubility of resveratrol, thereby greatly affecting the antioxidant activity of the drug. Additionally, resveratrol loaded SLN were synthesized with a controlled release profile, due to an initial burst release of 40% caused by the active principle associated with the particle shell, and a subsequent prolonged release of the drug located in the lipid matrix. In this system, the efficiency of the cellular uptake depended on the molecular interactions with the biological membrane organization, lipid rafts and the actin cytoskeleton invaginations for the receptor mediated entrance [85]. Resveratrol loaded SLN have been also prepared by Pandita et al. with a drug incorporation efficiency of 89% and average diameter of 134 nm [86]. This drug delivery system showed prolonged release in vitro up to 120 h in a Wistar rat model, enhancing plasma bioavailability compared to free drug suspension. Finally, cyclodextrins-resveratrol complexes have been used to increase concentration of polyphenol in aqueous solution, while maintaining its biological activity. For example, spherical cyclodextrin-based nanosponges showed increasing solubility and stability, together with good drug encapsulation efficiency, compared to free RE [87]. Ellagitannin (ET) and ellagic acid (EAC) are active substances belonging to the phenolic class of tannins most present in pomegranate (Punica granatum), a fruit-bearing shrub that originated in the region from Iran to northern India [71]. Nanosized drug delivery systems protect these molecules inside the nanoparticulate core, thus preventing degradation and increasing bioavailability. For example, Bala et al. synthesized ellagic acid-loaded PLGA NP which showed a rapid initial release of EAC in pH 7.4 phosphate buffer, followed by a slower sustained release [88]. Further, the authors tested the influence of the stabilizers p-dimethylaminobenzaldehyde (DMAB) and polyvinyl alcohol (PVA) on size, loading efficacy, release kinetics in PBS, stability, cytotoxic activity and in situ intestinal permeability of these nanoparticles [88]. A similar study was conducted also on ellagic acid-loaded PLGA-polycaprolactone (PCL)NP [89]. Both investigations resulted in improved tannin bioavailability with potential therapeutic applications. In the last few decades, there has been considerable interest in the active compounds in turmeric called curcuminoids, among which the most abundant is curcumin (90%), studied for its antioxidant, anti-inflammatory, anticancer, antiviral, and antifungal properties [90]. However, a very small percentage of the orally administered curcumin is detected in blood plasma, while the vast majority is eliminated in the feces and the urine [67]. Several recent in vitro studies have suggested that the anti-inflammatory action of curcumin may be linked to its ability to limit the production of inflammatory mediators [91–93], inhibit extracellular matrix degradation and chondrocyte apoptosis [94], and act as an anti-oxidant by decreasing the over-production of reactive oxygen species [95]. Zhang et al. demonstrated in vivo that topical application of curcumin NP was efficacious not only in slowing osteoarthritis disease progression, but also on pain relief [96]. Using a post-traumatic osteoarthritis mouse model, they showed that the topical application of curcumin encapsulated in hydrogel/glass based NP preserves the chondroprotective activity of curcumin, and may increase its bioavailability (Figure 4). Int. J. Mol. Sci. 2017, 18, 709 7 of 23 Int. J. Mol. Sci. 2017, 18, 709 7 of 23 Int. J. Mol. Sci. 2017, 18, 709 7 of 23 Int. J. Mol. Sci. 2017, 18, 709 7 of 23 Figure 4. Topical application of nanoencapsulated curcumin slowed the progression of Figure 4. Topical application of nanoencapsulated curcumin slowed the progression of osteoarthritis osteoarthritis induced by destabilization of the medial meniscus (DMM) in mice. Mice with DMM induced destabilization of topical the medial meniscus mice. Mice with DMM were werebytreated daily with application of (DMM) curcuminin nanoparticles or vehicle. Mice treated treated daily with topically topical application of curcumin nanoparticles or vehicle. Mice treated topically with curcumin with curcumin nanoparticles (Nano-C) exhibited improved Safranin O staining (A); lower nanoparticles (Nano-C) exhibited improved Safranin staining Osteoarthritis Osteoarthritis Research Society International (OARSI)Oscores (B); (A); and lower reduced synovitis (C); Research and Figure 4. International Topical application nanoencapsulated curcumin slowed the(C); progression subchondral bone (OARSI) plate ofthickness eight weeks after surgery compared toof that in vehicle Society scores(D) (B);atand reduced synovitis and subchondral bone plate osteoarthritis induced by destabilization of the medial meniscus (DMM) in mice. Mice with DMM control (Veh) (* p <weeks 0.05, t-test, = 5/group). Reproduced from [96] (https://www.ncbi.nlm.nih.gov/ thickness (D) at eight afternsurgery compared to that in vehicle control (Veh) (* p < 0.05, t-test, were treated daily with topical application of curcumin nanoparticles or vehicle. Mice treated pmc/articles/PMC4891896). Figure 4. Topical application of nanoencapsulated curcumin slowed the progression of n = 5/group). Reproduced from [96] (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4891896). topically with curcumin nanoparticles (Nano-C) exhibited improved Safranin O staining (A); lower osteoarthritis induced by destabilization of the medial meniscus (DMM) in mice. Mice with DMM Osteoarthritis Research Society International (OARSI) scores (B); and reduced synovitis (C); and were treated topical an application of curcumin nanoparticles or vehicle. Mice treated Wangdaily et al.with reported increment in curcumin anti-inflammatory activity when formulated in subchondral bone plate thickness (D) at eight weeks after surgery compared to that in vehicle Wang et al. reported an increment in curcumin anti-inflammatory activity when formulated topically with curcumin nanoparticles (Nano-C) exhibited improved Safranin O staining (A); lowervastly oil in water (o/w) nanoemulsions [97]. Emulsion-based formulations have been employed in in control (Veh) (* p < 0.05, t-test, n = 5/group). Reproduced from [96] (https://www.ncbi.nlm.nih.gov/ Osteoarthritis Research Society International (OARSI) scores (B); and reduced synovitis (C); and oil pmc/articles/PMC4891896). inpharmaceutical, water (o/w) nanoemulsions Emulsion-based formulations have been vastly employed cosmetic and food[97]. industry to protect active ingredients against external factors, to subchondral bone plate thicknessand at eight weeks after compared to that in vehicle in pharmaceutical, cosmetic food industry to surgery protect active ingredients against external factors, enhance their stability, and (D) to mask bad odors or taste [98]. Liposomes have also been employed as control (Veh) (* p < 0.05, t-test, n = 5/group). Reproduced from [96] (https://www.ncbi.nlm.nih.gov/ Wang et al. reported an increment in curcumin anti-inflammatory activity when formulated nanovectors for curcumin and release [99].[98]. Encapsulation ofhave curcumin in liposomes, to enhance their stability, and encapsulation to mask bad odors or taste Liposomes alsoinbeen employed as pmc/articles/PMC4891896). oil in water (o/w)using [97]. Emulsion-based formulations have been employed obtained commercially available and lecithin, was[99]. reported by vastly Takahashi et al.in [100].inResults nanovectors fornanoemulsions curcumin encapsulation release Encapsulation of curcumin liposomes, pharmaceutical, cosmetic and food industry to protect active ingredients against external factors, to showed that encapsulated curcumin was characterized by increased bioavailability, faster Wang et al. reported an increment in curcumin anti-inflammatory activity when formulated in Results obtained using commercially available lecithin, was reported by Takahashi et al. [100]. showed enhance their stability, and and to mask bad absorption odors or taste have also been employed as pharmacokinetics better of[98]. theLiposomes drug inhave rats. Overall, Table 1 summarizes the oil in water (o/w) nanoemulsions [97]. Emulsion-based formulations been vastly employed in that encapsulated curcumin was characterized by increased bioavailability, faster pharmacokinetics nanovectors for curcumin encapsulation and release [99]. Encapsulation of curcumin in liposomes, nanosized delivery systems used for anti-inflammatory properties. pharmaceutical, cosmetic and food industry to polyphenols protect active with ingredients against external factors, to and better absorption of the drug in rats. Overall, Table summarizes nanosized obtained using commercially available lecithin, was reported by1 Takahashi et al.the [100]. Results delivery systems enhance their stability, and to mask bad odors or taste [98]. Liposomes have also been employed as showed that encapsulated curcumin was characterized by increased bioavailability, faster used for polyphenols with anti-inflammatory properties. nanovectors for curcuminTable encapsulation anddelivery release [99]. Encapsulation of curcumin in liposomes, 1. Nanosized systems for anti-inflammatory polyphenols. pharmacokinetics and better absorption of the drug in rats. Overall, Table 1 summarizes the obtained using commercially available lecithin, was reported by Takahashi et al. [100]. Results Bioactive Principle Nanovector Type of Delivery System Experimental Model Reference nanosized delivery systems used for polyphenols with anti-inflammatory properties. Table 1.curcumin Nanosized delivery systems anti-inflammatory polyphenols. showed that encapsulated was characterized by increased bioavailability, faster NP offor soya lecithin, In vivo (rats) [76] and PEG Table 1 summarizes the pharmacokinetics and better absorption of the drug in Tween-80 rats. Overall, Quercetin Table 1. Nanosized delivery systems for anti-inflammatory polyphenols. PLGA NP Bioactive Principle Type of Delivery System Experimental Model nanosized delivery systems used forNanovector polyphenols with anti-inflammatory properties. In vitro [78] Reference quercetin Nanovector Nanoparticles Type of Delivery containing Experimental Model Reference NPSystem of soya lecithin, alcohol In vivo (rats) NP of soya Eudragit-polyvinyl lecithin, In vitro [79] In vivo (rats) [76] Tween-80 and PEG Table 1. Nanosized delivery systems for anti-inflammatory polyphenols. NP Tween-80 and quercetin-loaded PEG Quercetin Quercetin PLGA NP Quercetin-PLGA NP In vivo (rats) [80] PLGA NP Bioactive Principle Nanovector Type of Delivery System Experimental Reference In vitroModel [78] In vitro Nanoparticlescontaining quercetin Nanoparticles quercetin Lipid-coated NC NP of soyacontaining lecithin, Nanocapsules In vitro [77,78] In vivo (rats) [76] Eudragit-polyvinylQuercetin-PLGA alcohol NC In vitro Tween-80 and PEG [79] Eudragit-polyvinyl alcohol Quercetin quercetin-loaded NP PLGA NP In vitro In vivo PLGAquercetin-loaded NP NP [82] In vitro [78] Quercetin-PLGA NP In vivo (rats) [80] containing quercetin containing resveratrol (Wistar male rats) Nanoparticles Resveratrol Lipid-coated NC Quercetin-PLGA NP In vivovivo (rats) Eudragit-polyvinylEudragit alcohol RL 100 NP In vitro In vitro/In [83] NanocapsulesNanoparticles [77,78] In vitro [79] Quercetin-PLGA NC quercetin-loaded NP Lipid-coated NC Carboxymethyl PLGA NP NP In vivo vitro vivo (rats) [84] NanocapsulesQuercetin-PLGA In vivo (rats) In vitro/InIn [80] [82] chitosan NP Quercetin-PLGA (Wistar NC male rats) containing resveratrol Lipid-coated NC Resveratrol SLN with a controlled Nanocapsules In vitrovivo [77,78] Nanoparticles Solid lipid Eudragit RL 100 NPNP In vitro/In [83] In vitro [85] PLGA containing Quercetin-PLGA NC In vivo (Wistar male rats) Carboxymethyl release profile nanoparticles PLGA NP resveratrol In vitro/In In vivo vivo (rats) [84] Resveratrol loaded SLN In vivo (Wistar[82] male rats) [86] chitosan NP containing resveratrol (Wistar male rats) Nanoparticles Eudragit 100 NP InInvitro/In vivo Resveratrol Resveratrol Cyclodextrins CD-basedRL nanosponges vitro [87] SLN with a controlled Nanoparticles Eudragit RL 100 NP In vitro/In [83] In vitrovivo [85] Solid lipid release profileEllagic acid-loaded Carboxymethyl Carboxymethyl nanoparticles Ellagic acid Nanoparticles In vitro [88] vitro/In vivo (rats) [84] In vitro/In vivo (rats) Resveratrol loaded SLNPLGA NP In In vivo (Wistar male rats) [86] chitosan NP chitosan NP Cyclodextrins CD-based nanosponges In vitro [87] SLN with a controlled [85] Solid lipid SLN with a controlledIn vitro Ellagic acid-loaded release profile In vitro Ellagic acid Nanoparticles In vitro [88] Solid lipid nanoparticles PLGA NP release profile Resveratrol loaded SLN In vivo (Wistar male rats) [86] nanoparticles Cyclodextrins CD-based nanosponges In vitro In vivo (Wistar [87] male rats) Resveratrol loaded SLN Ellagic acid-loaded Ellagic acid Nanoparticles In [88] vitro Cyclodextrins CD-based nanospongesIn vitro PLGA NP Bioactive Principle [76] [78] [79] [80] [77,78] [82] [83] [84] [85] [86] [87] Int. J. Mol. Sci. 2017, 18, 709 8 of 23 Table 1. Cont. Bioactive Int. J. Mol. Sci. 2017, 18,Principle 709 Nanovector acid Ellagic18, acid Int. J. Mol. Sci.Ellagic 2017, 709 Type of Delivery System Ellagic acid-loaded Ellagic PLGA NP acid-loaded PLGA NP Experimental 8 of Model 23 In vitro [88] Reference 8 of 23 In vitro [88] Nanoparticles Nanoparticles In vivo (rats with In vivo (rats with induced nephrotoxicity) induced nephrotoxicity)In ellagic acid NP PLGA-PCL ellagic acid NP PLGA-PCL ellagic acid NP PLGA-PCL Curcumin Curcumin Curcumin [89] vivo [89] (rats with induced nephrotoxicity) In vivo (rats with Hydrogel/glass [96] post-traumatic osteoarthritis) In vivo (rats with In vivo (rats with Hydrogel/glass Hydrogel/glass [96] post-traumatic osteoarthritis) o/w nanoemulsion post-traumatic osteoarthritis) Nanoemulsions containing curcumin In vitro [98] o/w nanoemulsion o/w nanoemulsion in the oilcurcumin phase Nanoemulsions containing In vitro [98] Nanoemulsions curcumin In vitro Lipid in the oilcontaining phase Lecithin liposomes In vivo (rats) [100] in the oil phase nanoparticles Lipid Lecithin liposomes In vivo (rats) [100] nanoparticles polyethylene glycol; PLGA, polylactic-co-glycolic acid; NC, nanocapsules; Nanoparticles Nanoparticles Nanoparticles [89] [96] [98] NP, nanoparticles; PEG, Lipid nanoparticles Lecithin liposomes In vivo (rats) [100] NP,solid nanoparticles; PEG, polyethylene glycol; PLGA, polylactic-co-glycolic acid; NC, nanocapsules; SLN, lipid nanoparticles; CD, cyclodextrins; PCL, polycaprolactone; o/w, oil in water. NP, nanoparticles; PEG, polyethylene glycol; PLGA, polylactic-co-glycolic acid; NC, nanocapsules; SLN, solid lipid SLN, solid lipid nanoparticles; CD, cyclodextrins; PCL, polycaprolactone; o/w, oil in water. nanoparticles; CD, cyclodextrins; PCL, polycaprolactone; o/w, oil in water. 3.2. Phytocannabinoids 3.2. Phytocannabinoids Phytocannabinoids are defined as the lipophilic substances deriving from the hemp plant, 3.2. Phytocannabinoids Phytocannabinoids are defined as the lipophilic substances from the hemp plant, Cannabis sativa L., (Cannabaceae). The cannabis extract contains more deriving than 460 compounds of which Cannabis sativa L., (Cannabaceae). The cannabis extract contains more than 460 compounds of which Phytocannabinoids areAmong defined as the theprimary lipophilic substances deriving from the hemp plant, around 70 are phytocannabinoids. these, psychoactive phytocannabinoid is around 70 are phytocannabinoids. these, the primary psychoactive phytocannabinoid is Δ-9-tetrahydrocannabinol, commonly Among known as Δ9-THC [101], extract while other analogs available are460 Cannabis sativa L., (Cannabaceae). The cannabis contains more than compounds of Δ-9-tetrahydrocannabinol, commonly known as Δ9-THC [101], while other analogs availableIn are cannabidiol (CBD), cannabigerol, Δ-9-tetrahydrocannabivarin, cannabidavirin and cannabinol. which around 70 are phytocannabinoids. Among these, the primary psychoactive phytocannabinoid cannabidiol (CBD),and cannabigerol, cannabidavirin cannabinol. ®, a marketed and particular, Δ9-THC CBD are theΔ-9-tetrahydrocannabivarin, main components of Sativex medicine for theIn ®, a marketed is ∆-9-tetrahydrocannabinol, commonly known as ∆9-THC [101], while other particular, Δ9-THC and CBD are the main components of Sativex medicine for theanalogs available treatment of cancer pain [102]. Phytocannabinoids act by modulating cannabinoid receptors, which treatment of cancer pain [102]. Phytocannabinoids act by modulating cannabinoid receptors, which and cannabinol. are cannabidiol (CBD), cannabigerol, ∆-9-tetrahydrocannabivarin, cannabidavirin are involved in fundamental physiological processes of central and autonomic nervous systems, ® , asystems, are in fundamental physiological of central autonomic nervous In involved particular, ∆9-THC and CBD areprocesses the main components of Sativex marketed medicine for immune, endocrine, reproductive and cardiovascular activity [103].and Moreover, phytocannabinoids immune, endocrine, reproductive and cardiovascular activitythe [103]. Moreover,Gphytocannabinoids link other receptors like the potential receptor, deorphanized protein-coupled the treatment of cancer painvanilloid [102]. type-1 Phytocannabinoids act by modulating cannabinoid receptors, link other like the potential vanilloid type-1 receptor, theT-type deorphanized G protein-coupled receptor, thereceptors peroxisome proliferator-activated receptors and the Ca2+ channel. All these which are involved in fundamental physiological processes of central and autonomic receptor, the peroxisome proliferator-activated receptors andresponse the T-type Ca2+ channel. All also these nervous systems, targets regulate the anti-nociceptive and anti-inflammatory (Figure 3) but are immune, endocrine, reproductive cardiovascular activity [103]. phytocannabinoids targets regulate the of anti-nociceptive andand anti-inflammatory response (Figure 3)Moreover, but are also involved in the control sleep [104], obesity [105], epilepsy [106], breast cancer cell migration [107], involved in the control of sleep [104], obesity [105], epilepsy [106], breast cancer cell migration [107], link other receptors like the potential vanilloid type-1 receptor, the deorphanized and lipid and glucose metabolism [108]. Further, innumerable therapeutic indications have been G protein-coupled and lipidfor and glucose metabolism [108].asFurther, innumerable therapeutic indications have been reported phytocannabinoids such analgesic, anticonvulsant, hypnotic, tranquilizer, receptor, the peroxisome proliferator-activated receptors and the T-type Ca2+ channel. All these reported for phytocannabinoids such as analgesic, anticonvulsant, hypnotic, tranquilizer, anesthetic, anti-inflammatory, antibiotic, antiparasite, antispasmodic, nausea relieving, appetite targets regulate the anti-nociceptive and anti-inflammatory response (Figure 3) but are also involved anesthetic,diuretic, anti-inflammatory, antiparasite, nausea extract relieving, stimulant, antitussive antibiotic, and expectorant [109].antispasmodic, However, cannabis is appetite today in the control ofantitussive sleep [104], obesity [105], epilepsy [106], breast cancer cell migration [107], stimulant, diuretic, and expectorant [109]. However, cannabis extract is today considered as a potential drug of abuse, and medicine uses nanotechnology to specifically target the andprinciple lipidasand glucose metabolism [108]. Further, innumerable therapeutic have been considered a potential drug of abuse, and medicine usesside nanotechnology to specifically target the active avoiding the psychotropic action and other effects. For example, Esposito etindications al. active principle avoiding the psychotropic action and other to side effects. Forphytocannabinoids example, Espositotranquilizer, etinal. reported phytocannabinoids such asaanalgesic, anticonvulsant, hypnotic, anesthetic, described thefor development and optimization of method encapsulate described the lipid development and optimization a method to encapsulate phytocannabinoids in nanostructured carriers [110]. Such vectorsofwere produced by a melt and ultrasonication anti-inflammatory, antibiotic, antiparasite, antispasmodic, nausea relieving, appetite stimulant, nanostructured lipid carriersto [110]. Such vectors were produced by a encapsulation melt and ultrasonication protocol specifically designed optimize nanoparticle recovery and drug efficiency diuretic, antitussive and expectorant [109]. However, cannabis extract is today considered as a protocol specifically nanoparticle recovery and drugcarrier encapsulation efficiency [110]. Similarly, Durandesigned Lobato to et optimize al. proposed a valuable oral delivery for cannabinoid potential drug of abuse, and uses nanotechnology to carrier specifically target the active principle [110]. Similarly, et medicine al. based proposed a valuable oral delivery cannabinoid derivatives againstDuran chronicLobato pain states on lipid NP formulations [111]. Suchfor vectors were avoiding the psychotropic action and other side effects. For example, Esposito et al. described the derivatives againstsolvent-emulsion chronic pain states based on and lipidoptimized NP formulations [111]. vectors were developed through evaporation, regarding the Such physicochemical developed through solvent-emulsion evaporation, and optimized regarding the physicochemical development and optimization of aunder method to encapsulate phytocannabinoids properties, long-term stability, and integrity simulated gastric conditions. In particular, the in nanostructured properties, long-term stability, and inclusion integrity simulated In for particular, selection of the lipid core and the ofproduced lecithin proved beconditions. key factors the finalthe lipid carriers [110]. Such vectors wereunder by gastric a to melt and ultrasonication protocol specifically selection of the lipid core and the inclusion of lecithin proved to be key factors for the final properties of encapsulation, integrity, and performance of the carriers [111]. The same authors designed to optimize nanoparticle recovery and drug encapsulation efficiency [110]. Similarly, properties of encapsulation, integrity,Inand performance the surface-modified carriers [111]. The same authors prepared surface modified PLGANP. particular, plainofand particles were Duran Lobato al. proposed oral delivery carrier for cannabinoid derivatives against prepared surface et modified PLGANP.a valuable In particular, surface-modified were successfully developed using a nanoprecipitation method, plain using and chitosan (CS), Eudragitparticles RS, lecithin chronic pain states based on lipid NP formulations [111]. Such vectors were developed through successfully developed using a nanoprecipitation method, using chitosan (CS), Eudragit RS, lecithin and vitamin E as surface modifying agents. The nanoparticles exhibited mean particle size and vitamin E as surface modifying agents. The nanoparticles exhibited mean particle size solvent-emulsion optimized regarding properties, long-term distributions in the rangeevaporation, of 253–344 nm,and spherical shape and controlledthe zetaphysicochemical potential values. High distributions in the range of 253–344 nm, spherical shape and controlled zeta lecithin values. values of entrapment efficiency were obtained for all the formulations, while and vitamin stability, and integrity under simulated gastric conditions. In potential particular, theHigh selection of the lipid values of entrapment efficiency were obtained all the formulations, while formulations lecithin and vitamin E-modified particles showed higher release rates for when compared to the for other [112]. of encapsulation, core and the inclusion of lecithin proved to be key factors the final properties E-modified showed release rates when compared to the other [112]. Hernán Pérezparticles de la Ossa et al. higher developed cannabidiol loaded PCL particles as aformulations suitable dosage integrity, of the cannabidiol carriers [111]. same prepared Hernán Pérezand de the laperformance Ossa et al. developed loaded The PCL particlesauthors as a These suitable dosage surface modified form, prepared by oil-in-water emulsion–solvent evaporation technique [113]. vectors PLGANP. In by particular, plainemulsion–solvent and surface-modified particles form, prepared the oil-in-water evaporation techniquewere [113].successfully These vectors developed using had high entrapment efficiency (around 100%). CBD dissolved in the polymeric matrix the a nanoprecipitation method, using chitosan (CS), Eudragit RS, lecithin andofvitamin E as surface modifying agents. The nanoparticles exhibited mean particle size distributions in the range of 253–344 nm, spherical shape and controlled zeta potential values. High values of entrapment efficiency Int. J. Mol. Sci. 2017, 18, 709 9 of 23 were obtained for all the formulations, while lecithin and vitamin E-modified particles showed higher release rates when compared to the other formulations [112]. Hernán Pérez de la Ossa et al. developed cannabidiol loaded PCL particles as a suitable dosage form, prepared by the oil-in-water emulsion–solvent evaporation technique [113]. These vectors had high entrapment efficiency (around 100%). CBD dissolved in the polymeric matrix of the microspheres was slowly released in vitro within ten days. The results suggested that PCL particles were an alternative delivery system for long-term cannabinoid administration [94]. Martín-Banderas et al. produced ∆9-THC-loaded PLGA Int. J. Mol. Sci. 2017, 18, 709 9 of 23 nanoparticles [114]. The nanoformulation was further improved by surface functionalization with CS andmicrospheres PEG, to optimize the pharmacokinetics. Encapsulation efficiency (around 95%)particles was not affected by was slowly released in vitro within ten days. The results suggested that PCL delivery system for long-term cannabinoid administration [94]. Martín-Banderas thewere typeanofalternative polymeric coating. However, CS and PEG coatings decreased and increased, respectively, al. produced Δ9-THC-loaded PLGA nanoparticles [114]. The formulations nanoformulation was further theet∆9-THC release kinetics. The safety of all PLGA-based was confirmed by in vivo improved by surface functionalization with CS and PEG, to optimize the pharmacokinetics. compatibility studies; moreover, protein adsorption tests suggested that PEGylation protected the Encapsulation efficiency (around 95%) was not affected by the type of polymeric coating. However, phytodrug against immune [114]. Table 2 the actsΔ9-THC as a summary of synthesized CS and PEG coatings decreasedprocesses and increased, respectively, release kinetics. The safety nanosized delivery systems forformulations phytocannabinoids. of all PLGA-based was confirmed by in vivo compatibility studies; moreover, protein adsorption tests suggested that PEGylation protected the phytodrug against immune processes [114]. Table 2 Table acts as2. a summary of delivery synthesized nanosized delivery systems for phytocannabinoids. Nanosized systems for anti-inflammatory phytocannabinoids. Table 2. NanosizedNanovector delivery systems for anti-inflammatory phytocannabinoids. Bioactive Principle Type of Delivery System Experimental Model Bioactive Principle ∆-9-Tetrahydrocannabinol Δ-9-Tetrahydrocannabinol Nanovector Type of Delivery System Experimental Model Reference Nanostructured Lipid nanoparticles In vitro Nanostructured lipid carriers Lipid nanoparticles In vitro [110] lipid carriers Lipid NP In vitro Lipid NP containing lecithin In vitro [111] containing lecithin PLGA NP In vitro/In vivo PLGA NP In vitro/In vivo [112] Nanoparticles loaded loaded Nanoparticles Cannabidiol Cannabidiol In vitro In vitro [113] PCL NPPCL NP Δ9-THC-loaded In vivo (immunocompetent [114] In vivo (immunocompetent PLGA NP ∆9-THC-loaded PLGA NPC57BL/6 mice) C57BL/6 mice) Reference [110] [111] [112] [113] [114] 3.3. Phytosterols 3.3. Phytosterols Phytosterols (plant sterols and stanols) are naturally occurring compounds ubiquitously found in vegetable oils, cereal grains, nuts, legumes, fruits, and vegetables. In particular, the term Phytosterols (plant sterols and stanols) are naturally occurring compounds ubiquitously found phytosterol refers to more than 200 different compounds among which sitosterol, campesterol, in sitostanol vegetableand oils, cereal grains, nuts, legumes, fruits, and vegetables. In particular, the term campestanol are the most diffused. Phytosterols have various bioactive properties phytosterol refers to more than effect 200 different compounds amongcholesterol which sitosterol, such as blood cholesterol-lowering via partial inhibition of intestinal absorption, campesterol, anti-atherogenic effects and,are particularly for β-sitosterol, immune have stimulating anti- properties sitostanol and campestanol the most diffused. Phytosterols variousand bioactive inflammatory activities. Furthermore, several have suggested theofbeneficial effects of plant absorption, such as blood cholesterol-lowering effect studies via partial inhibition intestinal cholesterol sterols against the development of different types of cancer, such as colorectal, breast and prostate anti-atherogenic effects and, particularly for β-sitosterol, immune stimulating and anti-inflammatory cancer [115]. Despite their structural similarity to cholesterol, their bioavailability is limited. In activities. Furthermore, several studies have suggested the beneficial effects of plant sterols against particular, absorption is less than 2% for phytosterols, while it is 30–60% for cholesterol. On this thebasis, development different typescan of cancer, suchcontribute as colorectal, breastthe and prostate cancer [115]. the use ofof nanotechnology dramatically improving phytosterols Despite their structural similarity to cholesterol, their is limited. phytosterols In particular, absorption pharmacokinetics. To this aim, Leong et al. prepared andbioavailability characterized water-soluble nanodispersions. In particular, he studied of fourfor different types of On organic is less than 2% for phytosterols, whilethe it effects is 30–60% cholesterol. thisphases basis, the use of (hexane, isopropyl alcohol, ethanol, and acetone), their ratio with the aqueous phase and the use of nanotechnology can dramatically contribute improving the phytosterols pharmacokinetics. To this conventional homogenization vs. high-pressure homogenization on the vectors obtained. aim, Leong et al. prepared and characterized water-soluble phytosterols nanodispersions. In particular, Phytosterol nanodispersions were finally produced by emulsification-evaporation using hexane he [116]. studied theeteffects of four different types of organic (hexane, isopropyl ethanol, Rossi al. synthesized phytosterols colloidal particlesphases using antisolvent precipitationalcohol, in andpresence acetone), their ratio with the aqueous phase and the use of conventional homogenization vs. of a non-ionic surfactant. The resulting colloidal particles had rod-like shape with some degree of crystallinity. Moreover, the colloidal dispersions had good stability ensured by surface high-pressure homogenization on the vectors obtained. Phytosterol nanodispersions were finally charge, due to the presence of water and hydroxyl groups on the particle and by steric phytosterols produced by emulsification-evaporation using hexane [116]. Rossi surface, et al. synthesized stabilization, due to the use of a non-ionic stabilizer [117]. Turk et al. produced stable suspensions colloidal particles using antisolvent precipitation in presence of a non-ionic surfactant. The resulting of submicron particles of phytosterol by rapid expansion of a supercritical solution into aqueous colloidal rod-like shapetowith some degree crystallinity. Moreover, solutionparticles using fourhad different surfactants impede growth and of agglomeration of the submicron the colloidal dispersions had good stability ensured by surface charge, due to the presence of water particles. The obtained sizes were about 500 nm and, in most cases, a bimodal particle sizeand hydroxyl distribution was obtained. Long-term stability studies indicated modest particle growth over groups on the particle surface, and by steric stabilization, due to the use of a non-ionic 12 stabilizer [117]. months Table 3stable showssuspensions examples of of nanosized delivery systems for anti-inflammatory Turk et al. [118]. produced submicron particles of phytosterol by rapid expansion phytosterols. of a supercritical solution into aqueous solution using four different surfactants to impede growth and agglomeration of the submicron particles. The obtained sizes were about 500 nm and, in most Int. J. Mol. Sci. 2017, 18, 709 10 of 23 cases, a bimodal particle size distribution was obtained. Long-term stability studies indicated modest 12 months [118]. Table 3 shows examples of nanosized delivery 10 of 23 systems for anti-inflammatory phytosterols. particle growth over Int. J. Mol. Sci. 2017, 18, 709 Table 3. Nanosized delivery systems for anti-inflammatory phytosterols. Bioactive Principle Table Bioactive Principle Nanovector delivery Type of Deliveryfor System Experimental Model Reference 3. Nanosized systems anti-inflammatory phytosterols. Nanovector Nanodispersion produced by emulsification-evaporation Type of Delivery System In vitro Nanodispersion produced by Colloidal particles using emulsification-evaporation In vitro Phytosterol Phytosterol antisolvent precipitation Nanodispersion Nanodispersion Colloidal particles using antisolvent precipitation Suspensions of submicron Suspensions of submicron particles of phytosterol particles of phytosterol [116] Experimental Model In vitro Reference In vitro [116] In vitro [117] In vitro [118] [117] [118] 3.4. Carbohydrates 3.4. Carbohydrates Carbohydrates are one of the major components of plants and are essential to the proper growth andCarbohydrates developmentare of one vegetables. In fact, carbohydrate components maketoup of the primary of the major components of plants and are essential the90% proper cell wall are critical to wall function. Three typescomponents of polysaccharides, i.e., growth andand development of vegetables. In fact, carbohydrate make up 90% of high the molecular primary cell wall and aremainly critical contribute to wall function. Three types of pectin, polysaccharides, i.e., high weight carbohydrates, to plant structure: hemicelluloses and cellulose. molecular weight carbohydrates, mainly contribute to plant structure: pectin, hemicelluloses and Pectins are most abundant in the plant primary cell walls and in the middle lamellae, and are cellulose. most abundant in the plant acid. primaryHemicelluloses cell walls and in the lamellae, and defined Pectins by theare presence of galacturonic andmiddle cellulose represent the pure are defined by the presence of galacturonic acid. Hemicelluloses and cellulose represent the pure structural portion of the plants and are differentiated by the fact that hemicelluloses are small, structural portion of the plants and are differentiated by the fact that hemicelluloses are small, branched carbohydrate compounds made of different monosaccharides, while cellulose is made branched carbohydrate compounds made of different monosaccharides, while cellulose is made of of long unbranched composed exclusively of held glucose, heldbytogether bybonds. hydrogen bonds. long unbranched fibrils fibrils composed exclusively of glucose, together hydrogen Moreover,hemicellulose hemicellulose synthesized theGolgi, Golgi,while whilecellulose celluloseisissynthesized synthesizedininthe the cytoplasm, Moreover, is issynthesized in inthe near the plasma membrane [119]. Many plant polysaccharides have a strong action. cytoplasm, near the plasma membrane [119]. Many plant polysaccharides have aanti-inflammatory strong antiinflammatory action. For example, such as Echinacea purpurea and Echinacea angustifolia (Asteraceae) For example, such as Echinacea purpurea and Echinacea angustifolia (Asteraceae) that are known for their that are known for their and immunostimulating skin repairing Aqueous fractions immunostimulating skin repairingand properties [120].properties Aqueous[120]. fractions obtained from the roots obtained from the roots of these asteraceae contain echinacin, a polysaccharide with promising antiof these asteraceae contain echinacin, a polysaccharide with promising anti-inflammatory activity inflammatory activity in the skin of mice subjected to croton oil induced inflammation. A pectin in the skin of mice subjected to croton oil induced inflammation. A pectin isolated from the aerial isolated from the aerial parts of Comarum palustre (Rosaceae), the comaruman, was found to possess parts of Comarum palustre (Rosaceae), theactivity. comaruman, was found possess immunomodulating and immunomodulating and anti-inflammatory In particular, the to galacturonan fragments anti-inflammatory activity. In particular, the galacturonan fragments obtained by partial hydrolysis of obtained by partial hydrolysis of pectin (with molecular weight up to 10 kDa) were able to decrease pectin molecular up toto10fibronectin kDa) weretoable to decrease vitrothetheparent adhesion of neutrophils in vitro(with the adhesion of weight neutrophils a greater extentinthan pectin [121,122]. Popov et a preventive of comaruman on aceticPopov acid-induced to fibronectin toal. a demonstrated greater extent than the effect parent pectin [121,122]. et al. colitis demonstrated a in mice [123].effect The oral of this pectin for two dayscolitis prior to of colitis reduces preventive of administration comaruman on acetic acid-induced ininduction mice [123]. The oral administration neutrophil infiltration and enhances colon-bound mucus compared with the vehicle-treated colitis of this pectin for two days prior to induction of colitis reduces neutrophil infiltration and enhances group. colon-bound mucus compared with the vehicle-treated colitis group. The sulfated polysaccharides like xylose, glucose, arabinose, galactose and galactosamine The sulfated tripartita polysaccharides likeshowed xylose,anti-inflammatory glucose, arabinose, galactose galactosamine present present in Artemisia (Asteraceae) properties dueand to their ability in Artemisia tripartita (Asteraceae) showed anti-inflammatory properties due to their ability to alter to alter macrophage function, neutrophil count, and complement fixation function [124]. However, macrophage function, neutrophil count, and complement fixation function [124]. However, the most the most recent studies on nano-delivery of anti-inflammatory carbohydrates deal with the fresh extract Aloe vera, succulent plant of native to northern Africacarbohydrates that has rapidlydeal spread across the extract of recentofstudies on anano-delivery anti-inflammatory with the fresh world [125].aIn particular,plant the fresh gel to mainly consists of water and mesophyll cells (0.9% Aloe vera, succulent native northern Africa that(99.1%) has rapidly spread across the world [125]. dry matter) with the predominant sugar represented by mannose as mannose-6-phosphate, In particular, the fresh gel mainly consists of water (99.1%) and mesophyll cells (0.9% dry matter) with followed by other sugars in varying concentrations. Overall, arabinose, xylose, mannose, galactose, the predominant sugar represented by mannose as mannose-6-phosphate, followed by other sugars and glucose account for 69.2% of the total [125]. Liposomes containing Aloe vera gel having intrinsic in varying concentrations. Overall, arabinose, xylose, mannose, galactose, and glucose account for anti-inflammatory action, and with particle size of 200 nm have been prepared by Takahashi et al. 69.2% of theskin totalcollagen [125]. Liposomes containing veracell gel having intrinsic anti-inflammatory action, to enhance synthesis and growth Aloe of skin lines [126]. Encapsulation and and with particle sizeextract of 200onnm have beenforprepared by Takahashi et al. enhance stabilization of Aloe vera cotton fabric wound dressing application hasto been recentlyskin collagen accounted [127]. Moreover, co-encapsulation of Aloe vera with curcumin enhanced the delivery synthesisfor and growth of skin cell lines [126]. Encapsulation and stabilization of Aloe vera extract of the phytocompounds and their antioxidant activity [128]. Esmaeili et al. prepared polyamide NC Moreover, on cotton fabric for wound dressing application has been recently accounted for [127]. co-encapsulation of Aloe vera with curcumin enhanced the delivery of the phytocompounds and their antioxidant activity [128]. Esmaeili et al. prepared polyamide NC containing Aloe vera by Int. J. Mol. Sci. 2017, 18, 709 11 of 23 Int. J. Mol. Sci. 2017, 18, 709 11 of 23 the emulsion diffusion technique. In particular, sebacoyl chloride, Aloe vera extract and olive oil containing Aloe vera the emulsion diffusion technique. particular, sebacoyl chloride, Aloe verasulfoxide, were dissolved inby a complex organic phase made ofInacetone, ethyl acetate and dimethyl extract and olive oil were dissolved in a complex organic phase made of acetone, ethyl acetate while diethylenetriamine was solubilized in water to form the aqueous phase. Tweenand and gelatin dimethyl sulfoxide, while diethylenetriamine was solubilized in water to form the aqueous phase. were used as stabilizers. Nanocapsule dimensions depended on the polymer to oil ratio, the relative Tween andofgelatin were and usedplant as stabilizers. Nanocapsule depended on the polymer to amount polymers extract and the use ofdimensions surfactants. Optimized conditions resulted in oilnanocapsules ratio, the relative amount of polymers and plant extract and the use of surfactants. Optimized of 115 nm [129]. Table 4 summarizes the available nanosized vectors for carbohydrates conditions resulted in nanocapsules of 115 nm [129]. Table 4 summarizes the available nanosized with anti-inflammatory activity derived from plants. vectors for carbohydrates with anti-inflammatory activity derived from plants. Table 4. Nano sized delivery systems for anti-inflammatory carbohydrates. Table 4. Nano sized delivery systems for anti-inflammatory carbohydrates. Bioactive Principle Bioactive Principle Nanovector Nanovector Mannose-6-phosphate Mannose-6-phosphate Liposomes Liposomes Nanocapsules Nanocapsules TypeType of Delivery System of Delivery System Experimental Experimental Model Reference Reference Model Liposomes containing Liposomes containing AloeAloe veravera gel gel Invitro vitro In [123] [123] Liposomes containing Liposomes containing Aloe gel veraco-encapsulated gel co-encapsulated Aloe vera curcumin withwith curcumin In Invitro vitro [125] [125] In vitro [126] Polyamide NC Polyamide NC In vitro [126] 3.5. Essential Oils 3.5. Essential Oils Essential oils (EOs) are hydrophobic, volatile, natural, complex compounds characterized by a oils (EOs) are hydrophobic, volatile, natural, complex compounds characterized by strong Essential odor, formed by aromatic plants as secondary metabolites. Traditional EOs extraction techniques, usuallyformed based on or hydro-distillation, date back to the middle ages, when theyextraction a strong odor, bysteam aromatic plants as secondary metabolites. Traditional EOs were developed in Arabic Spain. Known for their antibacterial [130] and fungicidal [131] activity, techniques, usually based on steam or hydro-distillation, date back to the middle ages, when they and fordeveloped their fragrance and flavor, are also employed in food [130] preservation [132] and[131] in activity, were in Arabic Spain. they Known for their antibacterial and fungicidal medicine analgesic, sedative, anti-inflammatory, and localpreservation anesthetic remedies [133]. and for as their fragrance and flavor, they are alsospasmolytic employed in food [132] and in medicine EOs have been extensively studied, and there is much literature regarding their desirable as analgesic, sedative, anti-inflammatory, spasmolytic and local anesthetic remedies [133]. EOs have properties, however they are also very sensitive to the effects of light, oxygen, humidity and high been extensively studied, and there is much literature regarding their desirable properties, however temperature, so that their applications are limited [134]. Novel technologies for the administration they are also very sensitive to the effects of light, oxygen, humidity and high temperature, so that of plant extracts may have remarkable advantages over conventional formulations in terms of their applications are limited [134]. Novel technologies for the administration of plant extracts may enhancement of solubility, bioavailability, protection from toxicity, enhancement of pharmacological have remarkable advantages conventional formulationsdistribution, in terms ofsustained enhancement of solubility, activity, enhancement of stability,over improved tissue macrophages delivery, bioavailability, protection toxicity, enhancement of pharmacological and protection from physical from and chemical degradation [135]. Parris et al. haveactivity, reportedenhancement the of stability, of improved macrophages sustained delivery, and method, protection from encapsulation oregano tissue and cassia pure EOs distribution, in corn zein NC via phase separation physicalinand chemical degradation [135].[136]. Parris et al. have reported the encapsulation of oregano resulting a fast and high yield procedure Reportedly, EOs loaded particles have limited digestibility the stomach, slow release in the and more rapidresulting release ininthe and cassiainpure EOs in corn zein NC via small phaseintestine, separation method, a large fast and high intestine. It is worth[136]. noticing how, in their encapsulated form, little if no interaction of the EO yield procedure Reportedly, EOs loaded particles have limited digestibility inwith the stomach, other components in the feed was found. Zein, a prolamine protein found in maize, known for its noticing slow release in the small intestine, and more rapid release in the large intestine. It is worth superior biodegradability and biocompatibility, has also been used to nanoencapsulate thymol and how, in their encapsulated form, little if no interaction of the EO with other components in the feed carvacrol EOs via liquid–liquid dispersion method [137]. One of the most promising methods for was found. Zein, a prolamine protein found in maize, known for its superior biodegradability and the solubilization and stabilization of natural active agents involves the use of cyclodextrins (CD) as biocompatibility, has also been used to nanoencapsulate thymol and carvacrol EOs via liquid–liquid carriers [138]. In aqueous solution, the slightly polar CD cavity is occupied by water molecules and dispersion method [137]. One of the most promising methods for the solubilization and stabilization therefore they can be readily substituted by appropriate “guest molecules”, which are less polar of natural active agentsetinvolves use of cyclodextrins (CD) as carriers Inofaqueous than water [139]. Cevallos al. have the performed studies on the encapsulation and[138]. release thymol solution, thecinnamaldehyde slightly polar from CD cavity is The occupied by water and they canthebe readily and CD [140]. importance of thismolecules work lays in the therefore studies concerning substituted by appropriate “guest molecules”, which are less polar than water [139]. Cevallos et al. influence of water adsorption by CDs on the release of encapsulated compounds. Results showed have performed studiesappropriated on the encapsulation and release thymol and flavors cinnamaldehyde from the relevance of selecting storage conditions forofhydrophobic encapsulated in CD [140]. CD for predicting the shelflays lifeinof products formulated with nanoencapsulated Theorimportance of this work thefunctional studies concerning the influence of water adsorption by CDs on compounds. Olivera et al. reported the preparation of alginate/cashew gum NP via spray-drying, the release of encapsulated compounds. Results showed the relevance of selecting appropriated storage aiming at the development of a biopolymer blend for encapsulation of Lippiasidoides essential oil,functional conditions for hydrophobic flavors encapsulated in CD or for predicting the shelf life of primarily composed of thymol (50–70%) [141]. In the last decades, encapsulation techniques have products formulated with nanoencapsulated compounds. Olivera et al. reported the preparation of frequently been supported by innovative controlled and triggered release. Stimuli-responsive alginate/cashew gum NP via spray-drying, aiming at the development of a biopolymer blend for materials have therefore been employed as shell material for capsules and vector for drugs and encapsulation of Lippiasidoides essential oil, primarily composed of thymol (50–70%) [141]. In the last decades, encapsulation techniques have frequently been supported by innovative controlled and triggered release. Stimuli-responsive materials have therefore been employed as shell material for Int. J. Mol. Sci. 2017, 18, 709 12 of 23 capsules and vector for drugs and other active agents [142]. Bizzarro et al. recently reported on the Int.Int. J. Mol. Sci. 2017, 18,18, 709 1212 of of 2323 J. Mol. Sci. 2017, 709 preparation of cumin and basil oil-loaded polyamide capsules able to release their cargo oil under other active agents [142]. Bizzarro etet al.al. recently onon the preparation ofof cumin and basil oilother active agents [142]. Bizzarro recentlyreported reported the preparation cumin and basil oil- capsules UV-light irradiation [143]. Photo-responsive as well as other stimuli-responsive polymeric loaded polyamide capsules able to release their cargo oil under UV-light irradiation [143]. Photoloaded polyamide capsules able to release their cargo oil under UV-light irradiation [143]. Photohave been vastly reviewed in the literature [144]. Table 5 summarizes the nanosized delivery systems responsive asas well stimuli-responsive polymeric capsules have reviewed inin the responsive wellasasother other stimuli-responsive polymeric capsules havebeen beenvastly vastly reviewed the proposed for anti-inflammatory essential oils. literature literature[144]. [144].Table Table5 5summarizes summarizesthe thenanosized nanosizeddelivery deliverysystems systemsproposed proposedforforantiantiinflammatory essential oils. inflammatory essential oils. Table 5. Nanosized delivery systems for anti-inflammatory essential oils. Table 5. 5. Nanosized delivery systems forfor anti-inflammatory essential oils. Table Nanosized delivery systems anti-inflammatory essential oils. Bioactive Principle Nanovector Type of Delivery System Experimental Model Bioactive Principle Bioactive Principle Oregano and Cassia EOEO Oregano and Cassia Thymol and carvacrol Thymol and carvacrol Thymol and carvacrol Oregano and Cassia EO Reference Nanovector of of Delivery System Model Nanovector Type Type Delivery System Experimental Experimental Model Reference Reference Nanoparticles Corn zein NP In vitro Nanoparticles Corn zein NPNP In In vitro [136] Nanoparticles Corn zein vitro [136] [136] Nanoparticles Nanoparticles Nanoparticles In In vitro vitro [137] [137] In vitro [137] Cinnamon and thyme EOEO Cyclodextrins Inclusion in in CD In In vitro Cinnamon and thyme Cyclodextrins Inclusion CD vitro Cinnamon and thyme EO Cyclodextrins Inclusion in CD LippiasidoidesEO (50–70% Thymol) gum NPNP In In vitro LippiasidoidesEO (50–70% Thymol) Nanoparticles Nanoparticles Alginate/cashew Alginate/cashew gum vitro LippiasidoidesEO (50–70% Thymol)Nanocapsules Nanoparticles Polyamide Alginate/cashew gum NP Cumin and basil EOEO NC In In vitro Cumin and basil Nanocapsules Polyamide NC vitro [140] [140] [141] [141] In vitro [143] [143] [141] In vitro [143] Cumin and basil EO Nanocapsules Corn zein NPNPzein NP Corn zein Corn Polyamide NC In vitro [140] 3.6. Terpenoids 3.6. Terpenoids Among Amonga awide widespectrum spectrumofofnatural naturalproducts, products,terpenoids terpenoidsplay playa asignificant significantrole roleininprevention prevention 3.6. Terpenoids and andtreatment treatmentofofanti-inflammatory anti-inflammatoryconditions. conditions.These Thesecompounds compoundsare areproduced producedasassecondary secondary Amongofof a different wide spectrum of natural terpenoids play a significant role in prevention and metabolites organisms, such lichens, asaswell metabolites different organisms, suchasproducts, asplants, plants,mosses, mosses,algae, algae, lichens, wellas asinsects, insects, microbes InInrecent ofofthe microbesand and marineorganisms organisms[145]. [145]. recentyears, years,the themolecular molecularbasis basis theanti-inflammatory anti-inflammatory treatment of marine anti-inflammatory conditions. These compounds are produced as secondary metabolites effect ofofplant-derived terpenoids has atatthe center ofofextensive research. Several studies effect plant-derived terpenoids hasbeen been thealgae, centerlichens, extensive research. Several studiesand marine of different organisms, such as plants, mosses, as well as insects, microbes demonstrated that terpenoid ingredients can suppress nuclear factor-κB (NF-κB) signaling, the demonstrated that terpenoid ingredients can suppress nuclear factor-κB (NF-κB) signaling, the organisms [145]. In recent years, the molecular basis of the anti-inflammatory effect of plant-derived major majorregulator regulatorininthe thepathogenesis pathogenesisofofinflammatory inflammatorydiseases diseasesand andcancer cancer[146]. [146].Terpenoids Terpenoidsare area a terpenoids has been at the center of extensive research. Several studies demonstrated that terpenoid very verydiverse diversegroup groupofofmolecules, molecules,with withextraordinary extraordinaryvarying varyingchemistry, chemistry,structure structureand andfunction. function. ingredients can suppress nuclear factor-κBthe (NF-κB) signaling, the major regulator in the pathogenesis These number Thesecompounds compoundsare areclassified classifiedaccording accordingtotothe numberofofisoprene isopreneunits unitsand andcarbon carbonatoms atoms[147]. [147]. of inflammatory diseases cancer [146]. Terpenoids are a very diverse groupp-of Notably, the terpenes includes D-limonene, squalene, linalool, artemisinin, Notably, thelist listofof terpenesand includes D-limonene, squalene,geraniol, geraniol, linalool, artemisinin, p- molecules, with extraordinary varying chemistry, structure and function. These compounds are classified cymene, thymol Targeted ofoftypically hydrophobic terpenoids has cymene, thymoland andβ-carotene. β-carotene. Targeteddelivery delivery typically hydrophobic terpenoids hasalso also been the subject ofof massive research, finding applications inin a wide variety ofof fields. Among natural been the subject massive research, finding applications a wide variety fields. Among natural according to the number of isoprene units and carbon atoms [147]. Notably, the list of terpenes terpenoids, squalene is one of the most widespread, present in olives, shark liver oil, wheat terpenoids, squalene is one of the most widespread, present in olives, shark liver oil, wheat germ includes D-limonene, squalene, geraniol, linalool, artemisinin, p-cymene, thymolgerm and β-carotene. and applications [148]. the andeven evenhuman humanskin skin cells,and andpossesses possessesversatile versatile applications [148]. Lacatusu etal.al.reported reported the research, Targeted delivery ofcells, typically hydrophobic terpenoids has alsoLacatusu been theetsubject of massive design designand andcharacterization characterizationofofsoft softlipid lipidnanocarriers nanocarriersbased basedononbioactive bioactivepumpkin pumpkinand andamaranth amaranth finding applications in a wide variety of fields. Among natural terpenoids, squalene is one of the most oils, oils,rich richininsqualene, squalene,forforco-encapsulation co-encapsulationand andco-delivery co-deliveryofofUV-A UV-Aand andUV-B UV-Bfilters filters(avobenzone (avobenzone widespread, present inThe olives, shark liver oil, reported wheat germ even human skin cells, and possesses andoctocrylene) [149]. group later the preparation ofofsqualene-based andoctocrylene) [149]. Thesame same group later reported theand preparation squalene-based versatile applications [148]. Lacatusu et al.delivery reported design and nanocarriers forforthe and ofofthe hydrophilic and lipophilic nanocarriers theencapsulation encapsulation anddrug drug delivery hydrophilic andcharacterization lipophilicactives, actives,of soft lipid nanocarriers based on bioactive pumpkin and amaranth rich in for squalene, co-encapsulation confirming protective and effect ofof squalene sensitive confirmingthe the protective andstabilizing stabilizing effect squaleneasoils, asprotective protective forhighly highlyfor sensitive molecules [150]. On other hand, squalene can also bebe employed asas active agent. Adamczak al.al. group later molecules [150]. On the other hand, squalene can also employed active agent. Adamczak et and co-delivery ofthe UV-A and UV-B filters (avobenzone andoctocrylene) [149]. The et same developed a method of preparation of polyelectrolyte multilayer capsules, containing squalene developed a method of preparation of polyelectrolyte multilayer capsules, containing squalene as reported the preparation of squalene-based nanocarriers for the encapsulation andasdrug delivery hydrophobic phase, byby membrane emulsification technique [151,152]. InIn that work, squalene served hydrophobic phase, membrane emulsification technique [151,152]. that work, squalene served of hydrophilic and lipophilic actives, confirming the protective and stabilizing effect of squalene as asasboth bothactive activeagent agentand andsolvent solventcargo cargoforforhydrophobic hydrophobicdrug. drug.Alongside Alongsideitsitsanti-inflammatory anti-inflammatory protective for highlybeen sensitive molecules [150]. On the other hand, squalene canhas also be employed as activity, activity,squalene squalenehas has beenreported reportedhaving havingininvitro vitrocytoprotective cytoprotectiveactivity activity[153], [153],and and hasbeen been active Adamczak et al. developed a method of preparation ofcarotenoid polyelectrolyte multilayer found effective forforalopecia areata treatment [154]. is isa acarotenoid showing good foundagent. effective alopecia areata treatment [154].Lycopene Lycopene showing good capsules, containing squalene as hydrophobic phase, by and membrane emulsification technique [151,152]. In that pharmacological properties including commonly found inin pharmacological properties includingantioxidant antioxidant andanti-inflammatory, anti-inflammatory, commonly found tomatoes, red carrots, watermelons, and other red fruits and vegetables AsAs such, lycopene tomatoes, red carrots, watermelons, and other red fruits and vegetables [155,156]. such, lycopene work, squalene served as both active agent and solvent cargo [155,156]. for hydrophobic drug. Alongside its has a limited systemic absorption due toto a very low aqueous solubility. overcome this drawback, has a limited systemic absorption due ahas very low aqueous solubility. To overcome this drawback, anti-inflammatory activity, squalene been reported havingToin vitro cytoprotective activity [153], lycopene nanosized delivery systems have been recently engineered. Butnariu and Giuchici lycopene nanosized delivery systems have been recently engineered. Butnariu and Giuchici showing and has been found effective for alopecia areata treatment [154]. Lycopene is a carotenoid developed developednanoemulsions nanoemulsionsbased basedononaqueous aqueouspropolis propolisand andlycopene, lycopene,which whichwere weretested testedasas good pharmacological properties including antioxidant and anti-inflammatory, commonly found protective protectiveagents agentsagainst againstacute acuteUV-A-induced UV-A-inducedinflammation inflammationononratratpaw. paw.AAbetter bettertherapeutic therapeuticeffect effect in tomatoes, red carrots, watermelons, andcoupled other red fruits andtime vegetables [155,156]. was noticed totoa astandard with extended was noticedcompared compared standardsuspension, suspension, coupled with extended timeinterval intervalof oftested tested As such, lycopene has a limited systemic absorption due to a very low aqueous solubility. To overcome this drawback, lycopene nanosized delivery systems have been recently engineered. Butnariu and Giuchici developed nanoemulsions based on aqueous propolis and lycopene, which were tested as protective agents against acute UV-A-induced inflammation on rat paw. A better therapeutic Int. J. Mol. Sci. 2017, 18, 709 13 of 23 effect was noticed compared to a standard suspension, coupled with extended time interval of tested parameters [157]. More recently, stable lycopene-loaded SLN based on myristic acid or orange wax have been described, highlighting that chemical stability of lycopene entrapped in the SLN was Int. J. Mol. Sci. 2017, 18, 709 13 (CD) of 23 constitute significantly enhanced [158,159]. As reported in the previous sections, cyclodextrins Int. J. Mol. Sci. 2017, 18, 709 13 of 23 one of the most reliable nanocarriers for hydrophobic molecules, such as essential oils and Int. J. Mol. Sci. 2017, 18, 709 13 of 23 terpenoids. Int. J. Mol. Sci. 2017, 709 recently, stable lycopene-loaded SLN based on myristic acid or orange 13 wax of 23 parameters [157].18,More Forparameters example,[157]. a method to load lycopene into maltodextrin and cyclodextrin hasorbeen recently Morehighlighting recently, stable lycopene-loaded SLN based on myristic acid orange wax proposed have been described, thatlycopene-loaded chemical stability of based lycopene entrapped inorthe SLN wax was parameters [157]. More recently, stable SLN oneffect myristic acid orange bysignificantly Seo et al. [160]. The comparison of the anti-inflammatory of nanoencapsulated and free parameters [157]. Morehighlighting recently, As stable lycopene-loaded SLN on myristic acidinorthe orange have been described, that chemical stability of based lycopene entrapped SLN wax was enhanced [158,159]. reported in thestability previous have been described, highlighting that chemical ofsections, lycopenecyclodextrins entrapped in(CD) the constitute SLN was have been highlighting chemical stability of sections, lycopene entrapped in(CD) thelycopene SLN lycopene ondescribed, macrophage cell lines demonstrated that nanoencapsulation of significantly enhanced [158,159]. Asthat reported in the previous cyclodextrins constitute one of the most reliable nanocarriers for hydrophobic such as essential oils was and can further significantly enhanced [158,159]. As reported in the previousmolecules, sections, cyclodextrins (CD) constitute significantly enhanced [158,159]. As reported in the previous sections, cyclodextrins (CD) constitute one of its theanti-inflammatory most reliable nanocarriers forlycopene hydrophobic molecules, such as essential oils been and terpenoids. For example, a method to load into maltodextrin and cyclodextrin has improve effect during tissue-damaging inflammatory conditions. one of the most reliable nanocarriers for hydrophobic molecules, such as essential oils and one of interaction the most reliable forp-cymene, hydrophobic molecules, such as essential effect oils been and terpenoids. For example, a nanocarriers method to load lycopene into maltodextrin and cyclodextrin has recently proposed by Seo et al. [160]. The comparison of the anti-inflammatory of The between β-CD and one of the main anti-inflammatory constituents of terpenoids. For example, a method to load lycopene into maltodextrin and cyclodextrin has been terpenoids. For example, alycopene method to load lycopene into maltodextrin andthat cyclodextrin has been recently proposed byfree Seo et al. on [160]. The comparison of the anti-inflammatory effect of nanoencapsulated and macrophage cell lines demonstrated nanoencapsulation thyme andproposed cumin EOs, been extensively studied of [161,162]. Encapsulationeffect of p-cymene in CD recently by has Seo also et al. [160]. The comparison the anti-inflammatory of recently proposed byfree Seolycopene et its al. anti-inflammatory [160]. The comparison of thetissue-damaging anti-inflammatory effect of nanoencapsulated and on macrophage celleffect linesduring demonstrated that nanoencapsulation of lycopene can further improve inflammatory nanoencapsulated and reported free lycopene on macrophage cell linesThis demonstrated that nanoencapsulation has been successfully by Quintans et al. [163]. study involved the oral administration of nanoencapsulated and free lycopene on macrophage celleffect lines during demonstrated that nanoencapsulation of lycopene can further improve its anti-inflammatory tissue-damaging inflammatory conditions. of lycopene caninclusion further improve its anti-inflammatory effect during tissue-damaging inflammatory inclusion p-cymene/CD complexes to inhibit mice edema. Interestingly, p-cymene/β-CD of lycopene can further improve its and anti-inflammatory tissue-damaging inflammatory conditions. The interaction between β-CD p-cymene, oneeffect of theduring main anti-inflammatory constituents conditions. conditions. provided and EOs, morehas powerful same of freeofp-cymene at early stage, The faster interaction between β-CDbeen andinhibition p-cymene, than one ofthe the main amount anti-inflammatory constituents of thyme and cumin also extensively studied [161,162]. Encapsulation p-cymene in The interaction between β-CD and p-cymene, one of the main anti-inflammatory constituents The interaction between β-CD and p-cymene, one ofal.the main This anti-inflammatory of thyme and cumin EOs, hasreported alsocan been extensively studied [161,162]. Encapsulation of constituents p-cymene in CD has been successfully by Quintans et [163]. study involved the oral indicating that CD nanocarriers improve p-cymene anti-nociceptive and anti-inflammatory effects. of thyme and cumin EOs, has also been extensively studied [161,162]. Encapsulation of p-cymene in of thyme and cumin EOs, has also been extensively studied [161,162]. Encapsulation of p-cymene in CD has been successfully reported by Quintans et al. [163]. This study involved the oral administration of p-cymene/CD inclusion complexes to inhibit mice edema. Interestingly, pOther authors thereported encapsulation of other terpenoids CD. Menezes discussed the CD has been reported successfully by Quintans et al. [163]. Thisinstudy involved et theal.oral CD has beeninclusion successfully reported by et to al. inhibition [163]. This involved theof oral administration of p-cymene/CD inclusion complexes inhibit mice edema. Interestingly, pcymene/β-CD provided andQuintans more powerful thanstudy the same amount free inclusion complex of β-CD andfaster (−)-linalool [164], to a monoterpene alcohol compound administration of p-cymene/CD inclusion complexes inhibit mice edema. Interestingly, p-prevalent in administration of stage, p-cymene/CD inclusion complexes to can inhibit mice edema. Interestingly, pcymene/β-CD inclusion provided faster and more powerful inhibition than the same amount of free p-cymene at early indicating that CD nanocarriers improve p-cymene anti-nociceptive cymene/β-CD provided faster andspecies more powerful inhibition than the same amount free essential oils ofinclusion various aromatic plant [165]. can Other studies, carried out byof cymene/β-CD inclusion provided faster and powerful inhibition than the same amount ofQuintans-Júnior, free p-cymene at early stage, indicating that CDmore nanocarriers improve p-cymene anti-nociceptive and anti-inflammatory effects. Other authors reported the encapsulation of other terpenoids in CD. p-cymene at early stage, indicating that CD nanocarriers can improve p-cymene anti-nociceptive confirmed that ( − )-linalool-CD complexes produced superior anti-nociceptive effect, p-cymene at early stage, indicating that CD nanocarriers can improve p-cymene anti-nociceptive and anti-inflammatory effects. Other authors reported the encapsulation of other terpenoids in CD.with respect Menezes et al discussed the inclusion complex of β-CD and (−)-linalool [164],terpenoids a monoterpene and anti-inflammatory effects. Other authors reported the encapsulation of other in CD. toalcohol thatanti-inflammatory ofcompound (−al )-linalool alone, in experimental pain protocols [166]. In analogy with and effects. Other authors reported the encapsulation of other terpenoids in CD. Menezes et discussed the inclusion complex of β-CD and (−)-linalool [164], a monoterpene prevalent essential complex oils of various aromatic plant species [165].a Other studies, this result, Menezes et al discussed theininclusion of β-CD and (−)-linalool [164], monoterpene Menezes et al discussed the inclusion complex of β-CD and (−)-linalool [164], a monoterpene alcohol compound prevalent in essential oils of various aromatic plant species [165]. Other studies, Guimarães etbyal.Quintans-Júnior, reported the encapsulation of carvacrol, a terpenoid phenol present in the carried confirmed that (−)-linalool-CD complexes produced superior antialcohol out compound prevalentthat in essential oils of various aromatic plant species [165]. Other studies, alcohol compound prevalent in essential oils of various aromatic plant species [165]. Other studies, carried out by Quintans-Júnior, confirmed that (−)-linalool-CD complexes produced superior antinociceptive effect, with respect to that of (−)-linalool alone, in experimental pain protocols [166]. Incancer pain essential oilbyofQuintans-Júnior, oregano, with β-CD, that improves the pharmacological response carried out confirmed (−)-linalool-CD complexes produced superior on anticarried out by Quintans-Júnior, confirmed that (−)-linalool-CD complexes of produced superior antinociceptive effect, with respect to that of (−)-linalool alone, in experimental pain protocols [166]. In analogy with this result, Guimarães et al. reported that the encapsulation carvacrol, a terpenoid experimental protocols [167]. to It that is worth mentioning the terpene-cyclodextrin inclusion systems nociceptive effect, with respect of (−)-linalool alone,that in experimental pain protocols [166]. In nociceptive effect, withessential respect to that of alone, in experimental protocols [166]. In analogypresent with this result, Guimarães etoregano, al.(−)-linalool reported that theimproves encapsulation ofpain carvacrol, a terpenoid phenol in the oil of with β-CD, the pharmacological response analogy with this result, Guimarães et al. reported that the encapsulation of carvacrol, a terpenoid have been vastly reviewed inoil literature [168]. Overall, Table 6the recaps the main nanosized delivery analogy with Guimarães al. reported that theimproves encapsulation of a terpenoid phenol present in result, the essential ofetoregano, with β-CD, pharmacological response on cancer painthis experimental protocols [167]. Itwith is worth thatpharmacological thecarvacrol, terpene-cyclodextrin phenol present in the essential oil of oregano, β-CD,mentioning improves the response systems for anti-inflammatory terpenoids. phenol present the oil ofreviewed oregano, with β-CD,mentioning improves the response on cancer pain in experimental protocols [167]. Itin isliterature worth thatpharmacological the terpene-cyclodextrin inclusion systems haveessential been vastly [168]. Overall, Table 6 recaps the main on cancer pain experimental protocols [167]. It is worth mentioning that the terpene-cyclodextrin on cancer systems pain experimental [167]. Itinisliterature worth mentioning that the terpene-cyclodextrin inclusion have been protocols vastly reviewed [168]. Overall, Table 6 recaps the main nanosized delivery systems anti-inflammatory terpenoids. inclusion systems have beenforvastly reviewed in literature [168]. Overall, Table 6 recaps the main Table 6. Nanosized delivery systems for anti-inflammatory terpenoids. inclusion systems have been vastly reviewed in literature [168]. Overall, Table 6 recaps the main nanosized delivery systems for anti-inflammatory terpenoids. nanosized delivery systems for anti-inflammatory terpenoids. nanosized delivery systems for anti-inflammatory Table 6. Nanosized delivery systemsterpenoids. for anti-inflammatory terpenoids. Table 6. Nanosized Nanovector delivery systemsType for anti-inflammatory terpenoids. Bioactive Principle of Delivery Systems Experimental Model Bioactive Principle Nanovector Typefor of Delivery Systems Experimental Table 6. Nanosized delivery systems anti-inflammatory terpenoids. Model Reference Table 6. Nanosized delivery systems for anti-inflammatory terpenoids. Squalene Squalene Bioactive Principle Nanovector Type of Delivery Systems Experimental Model Reference Bioactive Principle Nanovector Type Polyelectrolyte of Delivery Systems Experimental Model Reference Polyelectrolyte Squalene Nanocapsules In vitro Model [151] Bioactive Principle Nanovector Type of Delivery Systems Experimental Nanocapsules In vitroReference Squalene multilayer NC NC multilayer Polyelectrolyte Squalene Lycopene Lycopene Lycopene Lycopene Lycopene p-Cymene p-Cymene p-Cymene p-Cymene Int. J. Mol. Sci. 2017, 18, 709 p-Cymene Nanocapsules Nanocapsules Nanocapsules Nanoemulsions Nanoemulsions Nanoemulsions Nanoparticles Nanoemulsions Nanoemulsions Nanoparticles Nanoparticles Cyclodextrins Nanoparticles Nanoparticles Cyclodextrins Cyclodextrins Cyclodextrins Cyclodextrins Polyelectrolyte multilayer NC Polyelectrolyte multilayer NC Aqueous propolis multilayer NCpropolis Aqueous and lycopene Aqueous propolis and lycopene Aqueous propolis and SLN lycopene Aqueous propolis and lycopene SLN SLN and lycopene Inclusion SLNin CD SLNin CD Inclusion Inclusion Inclusion in CD in CD Inclusion in CD In vitro [151] In vitro [151] vivo In vitro [151] In vivo [157] (albinoIn guinea vivo pigs) [157] (albino guinea[158,159] pigs) In vivo vitro (albinoIn [157] Inguinea vivo pigs) (albino guinea pigs) [157] In vitro [158,159] Inguinea vitro (albinoIn pigs) [160] In vitro vitro [158,159] In In vitro vitro In vitro[158,159] [160] In vitro [160] In vitro [160] Cyclodextrins Inclusion in CD In vitro 12 of 23 [162,163] Cyclodextrins Inclusion in CD In vitro [162,163] Cyclodextrins Cyclodextrins Cyclodextrins Cyclodextrins Inclusion in CD Inclusion in CD Inclusion in CD in CD Inclusion Cyclodextrins in CD in CD In and vitro basil Cyclodextrins In vitro other active agents [142]. Bizzarro et al. recently reported onInclusion theInclusion preparation of cumin oil-[162,163] Cyclodextrins Inclusion in CD In vitro [162,163] loaded polyamide (−)-Linalool capsules able to release their cargo oil under UV-light irradiation [143]. Photoresponsive as well as other stimuli-responsive polymeric capsules have been vastly reviewed in the (−)-Linalool (−)-Linalool literature [144]. Table 5 summarizes the nanosized delivery systems proposed for anti(−)-Linalool (− )-Linalool Cyclodextrins Inclusion in CD In vivo (rodents) [164,166] inflammatory essential oils. In vivo (rodents) [164,166] In vivo (rodents) [164,166] In vivo (rodents) [164,166] In vivo (rodents) Table 5. Nanosized delivery systems for anti-inflammatory essential oils. Carvacrol Bioactive Principle Carvacrol Carvacrol Oregano and Cassia EO Carvacrol Thymol and carvacrol Carvacrol Nanovector Type of Delivery System Experimental Model Reference Nanoparticles Corn zein NP In vitro [136] In vivo (rodents with Cyclodextrins Inclusion in CD [167] induced tumors) In vivo (rodents with Cyclodextrins Inclusion in CD [167] In vivo (rodents with tumors) Cyclodextrins Inclusion in CD [167] In induced vivo (rodents with induced tumors) Cyclodextrins Inclusion in CD [167] In vivo (rodents with NanoparticlesCyclodextrins Corn zein NP Inclusion inIn vitro induced tumors) [137] CD induced tumors) Cinnamon and thyme EO LippiasidoidesEO (50–70% Thymol) Cumin and basil EO Cyclodextrins Nanoparticles Nanocapsules Inclusion in CD Alginate/cashew gum NP Polyamide NC In vitro In vitro In vitro [140] [141] [143] 3.6. Terpenoids Among a wide spectrum of natural products, terpenoids play a significant role in prevention and treatment of anti-inflammatory conditions. These compounds are produced as secondary Reference [151] [157] [158,159] [160] [162,163] [164,166] [167] Int. J. Mol. Sci. 2017, 18, 709 Int. J. Mol. Sci. 2017, 18, 709 14 of 23 14 of 23 4. Conclusions 4. Conclusions Nowadays,phytochemicals phytochemicals have have promising promising potential potential to Nowadays, to prevent prevent and/or and/ortreat treatinflammatory inflammatory diseases. However,poor poorwater watersolubility, solubility,stability stability and and bioavailability, side effects diseases. However, bioavailability,together togetherwith withthe the side effects reported when used in therapy, have limited their clinical application so far. Nanosized drug reported when used in therapy, have limited their clinical application so far. Nanosized drug delivery systems increase the solubility and stability of phytochemicals, enhance their delivery systems can can increase the solubility and stability of phytochemicals, enhance their absorption, absorption, protect them from premature enzymatic degradation or metabolism in the body, protect them from premature enzymatic degradation or metabolism in the body, and prolongand their prolong their circulation time, limiting their side effects. A schematic diagram of the effects of circulation time, limiting their side effects. A schematic diagram of the effects of nanoparticle-delivered nanoparticle-delivered phytocompounds on the main cellular pathways involved in inflammation phytocompounds on the main cellular pathways involved in inflammation is reported in Figure 5. is reported in Figure 5. Nanotechnology represents a promising approach, which has already provided significant steps Nanotechnology represents a promising approach, which has already provided significant forward to bringing anti-inflammatory phytochemicals much closer to clinical applications. In this steps forward to bringing anti-inflammatory phytochemicals much closer to clinical applications. In frame, the increase in the number of nanotoxicological studies in the literature is related to a growing this frame, the increase in the number of nanotoxicological studies in the literature is related to a awareness sensibility ethics the of nanotechnology. Additional research research is needed growing and awareness and towards sensibilitythe towards ethics of nanotechnology. Additional is to improve the cost-effectiveness and long-term safety of nanosized drug delivery systems, through needed to improve the cost-effectiveness and long-term safety of nanosized drug delivery systems, thethrough use of biocompatible materials materials and un-harmful release processes, aimed to establish a stronger the use of biocompatible and un-harmful release processes, aimed to establish a correlation smart nanoparticle design anddesign rigorous assessment for future applications stronger between correlation between smart nanoparticle andsafety rigorous safety assessment for future in biomedicine. applications in biomedicine. Figure Schematicdiagram diagramofofthe thephytocompounds phytocompounds effects involved Figure 5. 5. Schematic effects on onthe themain maincellular cellularpathways pathways involved inflammation. After After cell cell internalization internalization of nanovector, encapsulated areare in in inflammation. of aa nanovector, encapsulatedphytocompounds phytocompounds released intocytoplasm. cytoplasm. Their action is elicited via inhibition of nitric released into Theiranti-inflammatory anti-inflammatory action is elicited via inhibition ofoxide nitric(NO) oxide production by by nitric oxide synthase (iNOS); reduction of of arachidonic acid and (NO) production nitric oxide synthase (iNOS); reduction arachidonic acidmetabolites metabolites and prostaglandins through inhibition of the cyclooxygenase (COX) and Phospholipase A2 (PLA2) prostaglandins through inhibition of the cyclooxygenase (COX) and Phospholipase A2 (PLA2) pathways; and regulationofofnuclear nuclearfactor factor NF-κB NF-κB and and mitogen-activated (MAPKs) pathways; and regulation mitogen-activatedprotein proteinkinases kinases (MAPKs) pathways, which modulate the expression of proand anti-inflammatory mediators including pathways, which modulate the expression of pro- and anti-inflammatory mediators including cytokines, cytokines, chemokines, and adhesion molecules. chemokines, and adhesion molecules. Int. J. Mol. Sci. 2017, 18, 709 15 of 23 Acknowledgments: This work was supported by Progetto PON01_01802: “Sviluppo di molecole capaci di modulare vie metaboliche intracellulari redox-sensibili per la prevenzione e la cura di patologie infettive, tumorali, neurodegenerative e loro delivery mediante piattaforme nano tecnologiche”; PON01_02512: “Ricerca e sviluppo di bioregolatori attivi sui meccanismi epigenetici dei processi infiammatori nelle malattie croniche e degenerative”; PON03_00106: “Materiali Avanzati per la Ricerca ed il comparto Agroalimentare, Laboratorio Pubblico-Privato, MAReA”; and PRIN 2012-201288JKYY: “Nanotecnologie per variare i programmi di sviluppo osseo nella parete vasale per la prevenzione e trattamento delle patologie associate alla calcificazione ectopica arteriosa”. Author Contributions: Raffaele Conte, Anna Calarco and Pierfrancesco Cerruti conceived and designed the paper; Raffaele Conte and Valentina Marturano wrote the paper; and Gianfranco Peluso helped to evaluate and edit the manuscript. Conflicts of Interest: The authors declare no conflict of interest. Abbreviations DDS PPH NP NC SLN QC RE PLGA ET EAC PEG PVA DMAB PBS PCL THC CBD CS EO CD Drug delivery systems Polyphenols Nanoparticles Nanocapsules Solid lipid nanoparticles Quercetin Resveratrol Polylactic-co-glycolic acid Ellagitannin Ellagic acid Polyethylene glycol Polyvinyl alcohol p-dimethylaminobenzaldehyde Phosphate-buffered saline Polycaprolactone Tetrahydrocannabinol Cannabidiol Chitosan Essential oils Cyclodextrins References 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. Denko, C.W. A role of neuropeptides in inflammation. In Biochemistry of Inflammation; Whicher, J.T., Evans, S.W., Eds.; Kluwer Publisher: London, UK, 1992; pp. 177–181. Tabas, I.; Glass, C.K. Anti-inflammatory therapy in chronic disease: Challenges and opportunities. Science 2013, 339, 166–172. [CrossRef] [PubMed] Baum, C.L.; Arpey, C.J. Normal cutaneous wound healing: Clinical correlation with cellular and molecular events. Dermatol. Surg. 2005, 31, 674–686. [CrossRef] [PubMed] Guo, S.; Di Pietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [CrossRef] [PubMed] Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [CrossRef] [PubMed] Pakyari, M.; Farrokhi, A.; Maharlooei, M.; Ghahary, A. Critical role of transforming growth factor β in different phases of wound healing. Adv. Wound Care 2013, 2, 215–224. [CrossRef] [PubMed] Nurden, A.T. Platelets, inflammation and tissue regeneration. Thromb. Haemost. 2011, 105, S13–S33. [CrossRef] [PubMed] Nurden, A.T.; Nurden, P.; Sanchez, M.; Andia, I.; Anitua, E. Platelets and wound healing. Front. Biosci. 2008, 13, 3532–3548. [CrossRef] [PubMed] Oberyszyn, T.M. Inflammation and wound healing. Front. Biosci. 2007, 12, 2993–2999. [CrossRef] [PubMed] Velnar, T.; Bailey, T.; Smrkoli, V. The wound-healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [CrossRef] [PubMed] Int. J. Mol. Sci. 2017, 18, 709 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 16 of 23 Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [CrossRef] [PubMed] Ryan, G.B.; Majno, G. Acute inflammation. A review. Am. J. Pathol. 1977, 86, 183–276. [PubMed] Golia, E.; Limongelli, G.; Natale, F.; Fimiani, F.; Maddaloni, V.; Pariggiano, I.; Bianchi, R.; Crisci, M.; D’Acierno, L.; Giordano, R.; et al. Inflammation and cardiovascular disease: From pathogenesis to therapeutic target. Curr. Atheroscler. Rep. 2014, 16, 435. [CrossRef] [PubMed] Montecucco, F.; Liberale, L.; Bonaventura, A.; Vecchiè, A.; Dallegri, F.; Carbone, F. The Role of Inflammation in Cardiovascular Outcome. Curr. Atheroscler. Rep. 2017, 19, 11. [CrossRef] [PubMed] Amor, S.; Peferoen, L.A.; Vogel, D.Y.; Breur, M.; van der Valk, P.; Baker, D.; van Noort, J.M. Inflammation in neurodegenerative diseases—An update. Immunology 2014, 142, 151–166. [CrossRef] [PubMed] Chen, W.W.; Zhang, X.; Huang, W.J. Role of neuroinflammation in neurodegenerative diseases. Mol. Med. Rep. 2016, 13, 3391–3396. [CrossRef] [PubMed] Perretti, M.; Cooper, D.; Dalli, J.; Norling, L.V. Immune resolution mechanisms in inflammatory arthritis. Nat. Rev. Rheumatol. 2017, 13, 87–99. [CrossRef] [PubMed] Bostanci, N.; Bao, K. Contribution of proteomics to our understanding of periodontal inflammation. Proteomics 2017, 17. [CrossRef] [PubMed] Lambrecht, B.N.; Hamida, H. The immunology of asthma. Nat. Immunol. 2015, 16, 45–56. [CrossRef] [PubMed] Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [CrossRef] [PubMed] Zhong, J.; Gong, Q.; Mima, A. Inflammatory Regulation in Diabetes and Metabolic Dysfunction. J. Diabetes Res. 2017, 2017, 5165268. [CrossRef] Crusz, S.M.; Balkwill, F.R. Inflammation and cancer: Advances and new agents. Nat. Rev. Clin. Oncol. 2015, 12, 584–596. [CrossRef] [PubMed] Shalapour, S.; Karin, M. Immunity, inflammation, and cancer: An eternal fight between good and evil. J. Clin. Investig. 2015, 125, 3347–3355. [CrossRef] [PubMed] González García, A.; Patier de la Peña, J.L.; Ortego Centeno, N. Autoinflammatory diseases in adults. Clinical characteristics and prognostic implications. Rev. Clin. Esp. 2017, 217, 108–116. [CrossRef] [PubMed] Rubartelli, A. Autoinflammatory diseases. Immunol. Lett. 2014, 161, 226–230. [CrossRef] [PubMed] Ciccarelli, F.; Martinis, M.D.; Ginaldi, L. An Update on Autoinflammatory Diseases. Curr. Med. Chem. 2013, 21, 261–269. [CrossRef] Abu-Remaileh, M.; Bender, S.; Raddatz, G.; Ansari, I.; Cohen, D.; Gutekunst, J.; Musch, T.; Linhart, H.; Breiling, A.; Pikarsky, E.; et al. Chronic inflammation induces a novel epigenetic program that is conserved in intestinal adenomas and in colorectal cancer. Cancer Res. 2015, 75, 2120–2130. [CrossRef] [PubMed] Raghuraman, S.; Donkin, I.; Versteyhe, S.; Barrès, R.; Simar, D. The Emerging Role of Epigenetics in Inflammation and Immunometabolism. Trends Endocrinol. Metab. 2016, 27, 782–795. [CrossRef] [PubMed] Bjarnason, I.; Hayllar, J.; MacPherson, A.J.; Russell, A.S. Side effects of nonsteroidal anti-inflammatory drugs on the small and large intestine in humans. Gastroenterology 1993, 104, 1832–1847. [CrossRef] Schacke, H.; Docke, W.D.; Asadullah, K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol.Ther. 2002, 96, 23–43. [CrossRef] Gurib-Fakim, A. Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol. Aspects Med. 2006, 27, 1–93. [CrossRef] [PubMed] Bellik, Y.; Boukraâ, L.; Alzahrani, H.; Bakhotmah, B.; Abdellah, F.; Hammoudi, S.; Iguer-Ouada, M. Molecular mechanism underlying anti-inflammatory and anti-allergic activities of phytochemicals: An update. Molecules 2013, 18, 322–353. [CrossRef] [PubMed] Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 2015, 20, 21138–21156. [CrossRef] [PubMed] Pan, M.H.; Lai, C.S.; Ho, C.T. Anti-inflammatory activity of natural dietary flavonoids. Food Funct 2010, 1, 15–31. [CrossRef] [PubMed] Chuang, C.C.; McIntosh, M.K. Potential mechanisms by which polyphenol-rich grapes prevent obesity-mediated inflammation and metabolic diseases. Annu. Rev. Nutr. 2011, 31, 155–176. [CrossRef] [PubMed] Int. J. Mol. Sci. 2017, 18, 709 36. 37. 38. 39. 40. 41. 42. 43. 44. 45. 46. 47. 48. 49. 50. 51. 52. 53. 54. 55. 56. 17 of 23 Dong, J.; Zhang, X.; Zhang, L.; Bian, H.X.; Xu, N.; Bao, B.; Liu, J. Quercetin reduces obesity-associated ATM infiltration and inflammation in mice: A mechanism including AMPKα 1/SIRT1. J. Lipid Res. 2014, 55, 363–374. [CrossRef] [PubMed] Talero, E.; Avila-Roman, J.; Motilva, V. Chemoprevention with phytonutrients and microalgae products in chronic inflammation and colon cancer. Curr. Pharm. Des. 2012, 18, 3939–3965. [CrossRef] [PubMed] Andlujar, I.; Recio, M.C.; Giner, R.M.; Cienfuegos-Jovellanos, E.; Laghi, S.; Muguerza, B.; Rios, J.L. Inhibition of ulcerative colitis in mice after oral administration of a polyphenol-enriched cocoa extract is mediated by the inhibition of STAT1 and STAT3 phosphorylation in colon cells. J. Agric. Food Chem. 2011, 59, 6474–6483. [CrossRef] [PubMed] Fruet, A.C.; Seito, L.N.; Rall, V.L.M.; Di Stasi, L.C. Dietary intervention with narrow-leaved cattail rhizome flour (Typha angustifolia L.) prevents intestinal inflammation in the trinitrobenzenesulphonic acid model of rat colitis. BMC Complement. Altern. Med. 2012, 12, 62. [CrossRef] [PubMed] Orsi, P.R.; Seito, L.N.; Di Stasi, L.C. Hymenaea stigonocarpa Mart. ex Hayne: A tropical medicinal plant with intestinal anti-inflammatory activity in TNBS model of intestinal inflammation in rats. J. Ethnopharmacol. 2014, 151, 380–385. [CrossRef] [PubMed] Poulose, S.M.; Miller, M.G.; Shukitt-Hale, B. Role of walnuts in maintaining brain health with age. J. Nutr. 2014, 144, 561S–566S. [CrossRef] [PubMed] Davinelli, S.; Sapere, N.; Zella, D.; Bracale, R.; Intrieri, M.; Scapagnini, G. Pleiotropic protective effects of phytochemicals in Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2012, 2012, 386527. [CrossRef] [PubMed] Meng, B.; Li, J.; Cao, H. Antioxidant and antiinflammatory activities of curcumin on diabetes mellitus and its complications. Curr. Pharm. Des. 2013, 19, 2101–2113. [CrossRef] [PubMed] Ferlazzo, N.; Cirmi, S.; Calapai, G.; Ventura-Spagnolo, E.; Gangemi, S.; Navarra, M. Anti-Inflammatory Activity of Citrus bergamia Derivatives: Where Do We Stand? Molecules 2016, 21, 1273–1283. [CrossRef] [PubMed] Nichols, J.A.; Katiyar, S.K. Skin photoprotection by natural polyphenols: Anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [CrossRef] [PubMed] Neves, A.R.; Lucio, M.; Lima, J.L.; Reis, S. Resveratrol in medicinal chemistry: A critical review of its pharmacokinetics, drug-delivery, and membrane interactions. Curr. Med. Chem. 2012, 19, 1663–1681. [CrossRef] [PubMed] Shi, J.; Votruba, A.R.; Farokhzad, O.C.; Langer, R. Nanotechnology in drug delivery and tissue engineering: From discovery to applications. Nano Lett. 2010, 10, 3223–3230. [CrossRef] [PubMed] Li, Z.; Jiang, H.; Xu, C.; Gu, L. A review: Using nanoparticles to enhance absorption and bioavailability of phenolic phytochemicals. Food Hydrocoll. 2015, 43, 153–164. [CrossRef] Conte, R.; De Luca, I.; De Luise, A.; Petillo, O.; Calarco, A.; Peluso, G. New therapeutic potentials of nanosized phytomedicine. J. Nanosci. Nanotechnol. 2016, 16, 8176–8187. [CrossRef] Aqil, F.; Munagala, R.; Jeyabalan, J.; Vadhanam, M.V. Bioavailability of phytochemicals and its enhancement by drug delivery systems. Cancer Lett. 2013, 334, 133–141. [CrossRef] [PubMed] Huang, Q.; Yu, H.; Ru, Q. Bioavailability and delivery of nutraceuticals using nanotechnology. J. Food Sci. 2010, 75, R50–R57. [CrossRef] [PubMed] Couvreur, P. Nanoparticles in drug delivery: Past, present and future. Adv. Drug Deliv. Rev. 2013, 65, 21–23. [CrossRef] [PubMed] Nair, H.B.; Sung, B.; Yadav, V.R.; Kannappan, R.; Chaturvedi, M.M.; Aggarwal, B.B. Delivery of antiinflammatory nutraceuticals by nanoparticles for the prevention and treatment of cancer. Biochem. Pharmacol. 2010, 80, 1833–1843. [CrossRef] [PubMed] Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew. Chem. Int. Ed. 2014, 53, 12320–12364. [CrossRef] [PubMed] Boyer, C.; Priyanto, P.; Davis, T.P.; Pissuwan, D.; Bulmus, V.; Kavallaris, M.; Teoh, W.Y.; Amal, R.; Carroll, M.; Woodward, R.; et al. Anti-fouling magnetic nanoparticles for siRNA delivery. J. Mater. Chem. 2010, 20, 255–265. [CrossRef] Huang, K.Y.; Ma, H.L.; Liu, J.; Huo, S.D.; Kumar, A.; Wei, T.; Zhang, X.; Jin, S.; Gan, Y.; Wang, P.C.; et al. Size-dependent localization and penetration of ultrasmall gold nanoparticles in cancer cells, multicellular spheroids, and tumors in vivo. ACS Nano 2012, 6, 4483–4493. [CrossRef] [PubMed] Int. J. Mol. Sci. 2017, 18, 709 57. 58. 59. 60. 61. 62. 63. 64. 65. 66. 67. 68. 69. 70. 71. 72. 73. 74. 75. 76. 77. 78. 18 of 23 Steichen, S.D.; Caldorera-Moore, M.; Peppas, N.A. A review of current nanoparticle and targeting moieties for the delivery of cancer therapeutics. Eur. J. Pharm. Sci. 2013, 48, 416–427. [PubMed] Sagnella, S.M.; Conn, C.E.; Krodkiewska, I.; Moghaddam, M.; Seddon, J.M.; Drummond, C.J. Ordered nanostructured amphiphile self-assembly materials from endogenous nonionic unsaturated monoethanolamide lipids in water. Langmuir 2010, 26, 3084–3094. [PubMed] Sagnella, S.M.; Gong, X.J.; Moghaddam, M.J.; Conn, C.E.; Kimpton, K.; Waddington, L.J.; Krodkiewska, I.; Drummond, C.J. Nanostructured nanoparticles of self-assembled lipid pro-drugs as a route to improved chemotherapeutic agents. Nanoscale 2011, 3, 919–924. [PubMed] Challa, R.; Ahuja, A.; Ali, J.; Khar, R.K. Cyclodextrins in drug delivery: An updated review. AAPS Pharm. Sci. Technol. 2005, 6, E329–E357. [CrossRef] [PubMed] Cho, K.; Wang, X.U.; Nie, S.; Shin, D.M. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [CrossRef] [PubMed] Sagnella, S.M.; McCarroll, J.A.; Kavallaris, M. Drug delivery: Beyond active tumour targeting. Nanomedicine 2014, 10, 1131–1137. [CrossRef] [PubMed] Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [CrossRef] [PubMed] Verma, A.; Stellacci, F. Effect of surface properties on nanoparticle-cell interactions. Small 2010, 6, 12–21. [CrossRef] [PubMed] Pasche, S.; Voros, J.; Griesser, H.J.; Spencer, N.D.; Textor, M. Effects of ionic strength and surface charge on protein adsorption at pegylated surfaces. J. Phys. Chem. B 2005, 109, 17545–17552. [CrossRef] [PubMed] Singh, R.; Lillard, J.W. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [CrossRef] [PubMed] Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [CrossRef] [PubMed] Dobrovolskaia, M.A.; Shurin, M.; Shvedova, A.A. Current understanding of interactions between nanoparticles and the immune system. Toxicol. Appl. Pharmacol. 2016, 299, 78–89. [CrossRef] [PubMed] Shvedova, A.A.; Kagan, V.E.; Fadeel, B. Close encounters of the small kind: Adverse effects of man-made materials interfacing with the nano-cosmos of biological systems. Annu. Rev. Pharm. Toxicol. 2010, 50, 63–88. [CrossRef] [PubMed] Meng, H.; Xia, T.; George, S.; Nel, A.E. A predictive toxicological paradigm for the safety assessment of nanomaterials. ACS Nano 2009, 3, 1620–1627. [CrossRef] [PubMed] Conte, R.; Calarco, A.; Napoletano, A.; Valentino, A.; Margarucci, S.; di Cristo, F.; Di Salle, A.; Peluso, G. Polyphenols nanoencapsulation for therapeutic applications. J. Biomol. Res. Ther. 2016, 5, 2. Milbury, P.E.; Vita, J.A.; Blumberg, J.B. Anthocyanins are bioavailable in humans following an acute dose of cranberry juice. J. Nutr. 2010, 140, 1099–1104. [CrossRef] [PubMed] Gonzalez, R.; Ballester, I.; Lopez-Posadas, R.; Suarez, M.D.; Zarzuelo, A.; Martinez-Augustin, O.; Sanchez de Medina, F. Effects of flavonoids and other polyphenols on inflammation. Crit. Rev. Food Sci. Nutr. 2011, 51, 331–362. [CrossRef] [PubMed] D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [CrossRef] [PubMed] Etheridge, M.L.; Campbell, S.A.; Erdman, A.G.; Haynes, C.L.; Wolf, S.M.; McCullough, J. The big picture on nanomedicine: The state of investigational and approved nanomedicine products. Nanomedicine 2013, 9, 1–14. [CrossRef] [PubMed] Li, H.; Zhao, X.; Ma, Y.; Zhai, G.; Li, L.; Lou, H. Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J. Controll. Release 2009, 133, 238–244. [CrossRef] [PubMed] Barras, A.; Mezzetti, A.; Richard, A.; Lazzaroni, S.; Roux, S.; Melnyk, P.; Betbeder, D.; Monfilliette-Dupont, N. Formulation and characterization of polyphenol-loaded lipid nanocapsules. Int. J. Pharm. 2009, 379, 270–277. [CrossRef] [PubMed] Pool, H.; Quintanar, D.; Figueroa, J.D.D.; Marinho Mano, C.; Bechara, J.E.H.; Godinez, L.A.; Mendoza, S. Antioxidant effects of quercetin and catechin encapsulated into plga nanoparticles. J. Nanomater. 2012, 2012, 12. [CrossRef] Int. J. Mol. Sci. 2017, 18, 709 79. 19 of 23 Wu, T.H.; Yen, F.L.; Lin, L.T.; Tsai, T.R.; Lin, C.C.; Cham, T.M. Preparation, physicochemical characterization, and antioxidant effects of quercetin nanoparticles. Int. J. Pharm. 2008, 346, 160–168. [CrossRef] [PubMed] 80. Chakraborty, S.; Stalin, S.; Das, N.; Choudhury, S.T.; Ghosh, S.; Swarnakar, S. The use of nano-quercetin to arrest mitochondrial damage and mmp-9 upregulation during prevention of gastric inflammation induced by ethanol in rat. Biomaterials 2012, 33, 2991–3001. [CrossRef] [PubMed] 81. Goswami, S.K.; Das, D.K. Resveratrol and chemoprevention. Cancer Lett. 2009, 284, 1–6. [CrossRef] [PubMed] 82. Singh, G.; Pai, R.S. Optimized PLGA nanoparticle platform for orally dosed trans-resveratrol with enhanced bioavailability potential. Expert Opin. Drug Deliv. 2014, 11, 647–659. [CrossRef] [PubMed] 83. Singh, G.; Pai, R.S. In Vitro/in-vivo characterization of trans-resveratrol-loaded nanoparticulate drug delivery system for oral administration. J. Pharmacy Pharmacol. 2014, 66, 1062–1076. 84. Zu, Y.; Zhang, Y.; Wang, W.; Zhao, X.; Han, X.; Wang, K.; Ge, Y. Preparation and in vitro/in vivo evaluation of resveratrol-loaded carboxymethyl chitosan nanoparticles. Drug Deliv. 2016, 23, 981–991. [PubMed] 85. Teskac, K.; Kristl, J. The evidence for solid lipid nanoparticles mediated cell uptake of resveratrol. Int. J. Pharm. 2010, 390, 61–69. [CrossRef] [PubMed] 86. Pandita, D.; Kumar, S.; Poonia, N.; Lather, V. Solid lipid nanoparticles enhance oral bioavailability of resveratrol, a natural polyphenol. Food Res. Int. 2014, 62, 1165–1174. [CrossRef] 87. Ansari, K.A.; Vavia, P.R.; Trotta, F.; Cavalli, R. Cyclodextrin-based nanosponges for delivery of resveratrol: In vitro characterisation, stability, cytotoxicity and permeation study. AAPS Pharm. Sci. Tech. 2011, 12, 279–286. [CrossRef] [PubMed] 88. Bala, I.; Bhardwaj, V.; Hariharan, S.; Kharade, S.V.; Roy, N.; Ravi Kumar, M.N. Sustained release nanoparticulate formulation containing antioxidant-ellagic acid as potential prophylaxis system for oral administration. J. Drug Target 2006, 14, 27–34. [CrossRef] [PubMed] 89. Sonaje, K.; Italia, J.L.; Sharma, G.; Bhardwaj, V.; Tikoo, K.; Kumar, M.N. Development of biodegradable nanoparticles for oral delivery of ellagic acid and evaluation of their antioxidant efficacy against cyclosporine a-induced nephrotoxicity in rats. Pharm. Res. 2007, 24, 899–908. [CrossRef] [PubMed] 90. Chainani-Wu, N. Safety and anti-inflammatory activity of curcumin: A component of tumeric (Curcuma longa). J. Altern. Complement. Med. 2003, 9, 161–168. [CrossRef] [PubMed] 91. Goel, A.; Boland, C.R.; Chauhan, D.P. Specific inhibition of cyclooxygenase-2 (COX-2) expression by dietary curcumin in HT-29 human colon cancer cells. Cancer Lett. 2001, 172, 111–118. [CrossRef] 92. Mathy-Hartert, M.; Jacquemond-Collet, I.; Priem, F.; Sanchez, C.; Lambert, C.; Henrotin, Y. Curcumin inhibits pro-inflammatory mediators and metalloproteinase-3 production by chondrocytes. Inflamm. Res. 2009, 58, 899–908. [CrossRef] [PubMed] 93. Henrotin, Y.; Clutterbuck, A.L.; Allaway, D.; Lodwig, E.M.; Harris, P.; Mathy-Hartert, M.; Shakibaei, M.; Mobasheri, A. Biological actions of curcumin on articular chondrocytes. Osteoarthr. Cartil. 2010, 18, 141–149. [CrossRef] [PubMed] 94. Shakibaei, M.; Mobasheri, A.; Buhrmann, C. Curcumin synergizes with resveratrol to stimulate the MAPK signaling pathway in human articular chondrocytes in vitro. Genes Nutr. 2011, 6, 171–179. [CrossRef] [PubMed] 95. Sreejayan, N.; Rao, M.N. Free radical scavenging activity of curcuminoids. Arzneimittelforschung 1996, 46, 169–171. [PubMed] 96. Zhang, Z.; Leong, D.J.; Xu, L.; He, Z.; Wang, A.; Navati, M.; Kim, S.J.; Hirsh, D.M.; Hardin, J.A.; Cobelli, N.J.; et al. Curcumin slows osteoarthritis progression and relieves osteoarthritis-associated pain symptoms in a post-traumatic osteoarthritis mouse model. Arthritis Res. Ther. 2016, 18, 128–13. [CrossRef] [PubMed] 97. Wang, X.; Jiang, Y.; Wang, Y.-W.; Huang, M.-T.; Ho, C.-T.; Huang, Q. Enhancing anti-inflammation activity of curcumin through o/w nanoemulsions. Food Chem. 2008, 108, 419–424. [CrossRef] [PubMed] 98. Madene, A.; Jacquot, M.; Scher, J.; Desobry, S. Flavour encapsulation and controlled release—A review. Int. J. Food Sci. Technol. 2006, 41, 1–21. [CrossRef] 99. Li, L.; Braiteh, F.S.; Kurzrock, R. Liposome-encapsulated curcumin. Cancer 2005, 104, 1322–1331. [CrossRef] [PubMed] 100. Takahashi, M.; Uechi, S.; Takara, K.; Asikin, Y.; Wada, K. Evaluation of an oral carrier system in rats: Bioavailability and antioxidant properties of liposome-encapsulated curcumin. J. Agric. Food Chem. 2009, 57, 9141–9146. [CrossRef] [PubMed] Int. J. Mol. Sci. 2017, 18, 709 20 of 23 101. Elsohly, M.A.; Slade, D. Chemical constituents of marijuana: The complex mixture of natural cannabinoids. Life Sci. 2005, 78, 539–548. [CrossRef] [PubMed] 102. Hill, A.J.; Williams, C.M.; Whalley, B.J.; Stephens, G.J. Phytocannabinoids as novel therapeutic agents in cns disorders. Pharmacol. Therapeut. 2012, 133, 79–97. [CrossRef] [PubMed] 103. Di Marzo, V. Endocannabinoid system. In eLS; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2001. 104. Anderson, M.P.; Mochizuki, T.; Xie, J.; Fischler, W.; Manger, J.P.; Talley, E.M.; Scammell, T.E.; Tonegawa, S. Thalamic cav3.1 T-type Ca2+ channel plays a crucial role in stabilizing sleep. Proc. Natl. Acad. Sci. USA 2005, 102, 1743–1748. [CrossRef] [PubMed] 105. Moreno-Navarrete, J.M.; Catalan, V.; Whyte, L.; Diaz-Arteaga, A.; Vazquez-Martinez, R.; Rotellar, F.; Guzman, R.; Gomez-Ambrosi, J.; Pulido, M.R.; Russell, W.R.; et al. The l-α-lysophosphatidylinositol/gpr55 system and its potential role in human obesity. Diabetes 2012, 61, 281–291. [CrossRef] [PubMed] 106. Vitko, I.; Chen, Y.; Arias, J.M.; Shen, Y.; Wu, X.R.; Perez-Reyes, E. Functional characterization and neuronal modeling of the effects of childhood absence epilepsy variants of cacna1h, a T-type calcium channel. J. Neurosci. 2005, 25, 4844–4855. [CrossRef] [PubMed] 107. Ford, L.A.; Roelofs, A.J.; Anavi-Goffer, S.; Mowat, L.; Simpson, D.G.; Irving, A.J.; Rogers, M.J.; Rajnicek, A.M.; Ross, R.A. A role for l-alpha-lysophosphatidylinositol and gpr55 in the modulation of migration, orientation and polarization of human breast cancer cells. Br. J. Pharmacol. 2010, 160, 762–771. [CrossRef] [PubMed] 108. Jay, M.A.; Ren, J. Peroxisome proliferator-activated receptor (PPAR) in metabolic syndrome and type 2 diabetes mellitus. Curr. Diabetes Rev. 2007, 3, 33–39. [CrossRef] [PubMed] 109. Zuardi, A.W. History of cannabis as a medicine: A review. Rev. Bras. Psiquiatr. 2006, 28, 153–157. [CrossRef] [PubMed] 110. Esposito, E.; Drechsler, M.; Cortesi, R.; Nastruzzi, C. Encapsulation of cannabinoid drugs in nanostructured lipid carriers. Eur. J. Pharm. Biopharm. 2016, 102, 87–91. [CrossRef] [PubMed] 111. Durán-Lobato, M.; Martín-Banderas, L.; Lopes, R.; Gonçalves, L.M.D.; Fernández-Arévalo, M.; Almeida, A.J. Lipid nanoparticles as an emerging platform for cannabinoid delivery: Physicochemical optimization and biocompatibility. Drug Dev. Ind. Pharmacy 2016, 42, 190–198. [CrossRef] [PubMed] 112. Duran-Lobato, M.; Munoz-Rubio, I.; Holgado, M.A.; Alvarez-Fuentes, J.; Fernandez-Arevalo, M.; Martin-Banderas, L. Enhanced cellular uptake and biodistribution of a synthetic cannabinoid loaded in surface-modified poly(lactic-co-glycolic acid) nanoparticles. J. Biomed. Nanotechnol. 2014, 10, 1068–1079. [CrossRef] [PubMed] 113. Hernán Pérez de la Ossa, D.; Ligresti, A.; Gil-Alegre, M.E.; Aberturas, M.R.; Molpeceres, J.; Di Marzo, V.; Torres Suárez, A.I. Poly-ε-caprolactone microspheres as a drug delivery system for cannabinoid administration: Development, characterization and in vitro evaluation of their antitumoral efficacy. J. Control Release 2012, 161, 927–932. [CrossRef] [PubMed] 114. Martín-Banderas, L.; Muñoz-Rubio, I.; Prados, J.; Álvarez-Fuentes, J.; Calderón-Montaño, J.M.; López-Lázaro, M.; Arias, J.L.; Leiva, M.C.; Holgado, M.A.; Fernández-Arévalo, M. In vitro and in vivo evaluation of ∆9 -tetrahidrocannabinol/PLGA nanoparticles for cancer chemotherapy. Int. J. Pharm. 2015, 487, 205–212. [CrossRef] [PubMed] 115. Trautwein, E.A.; Demonty, I. Phytosterols: Natural compounds with established and emerging health benefits. OCL-Oleagineux Corps Gras Lipides 2010, 14, 259–266. [CrossRef] 116. Leong, W.-F.; Lai, O.-M.; Long, K.; Che Man, Y.B.; Misran, M.; Tan, C.-P. Preparation and characterisation of water-soluble phytosterol nanodispersions. Food Chem. 2011, 129, 77–83. [CrossRef] 117. Rossi, L.; Seijen ten Hoorn, J.W.M.; Melnikov, S.M.; Velikov, K.P. Colloidal phytosterols: Synthesis, characterization and bioaccessibility. Soft Matter 2010, 6, 928–936. [CrossRef] 118. Turk, M.; Lietzow, R. Stabilized nanoparticles of phytosterol by rapid expansion from supercritical solution into aqueous solution. AAPS Pharm. Sci. Tech. 2004, 5, e56. [CrossRef] [PubMed] 119. Caffall, K.H.; Mohnen, D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [CrossRef] [PubMed] 120. Tragni, E.; Galli, C.L.; Tubaro, A.; Del Negro, P.; Della Loggia, R. Anti-inflammatory activity of echinacea angustifolia fractions separated on the basis of molecular weight. Pharmacol. Res. Commun. 1988, 20 (Suppl. S5), 87–90. [CrossRef] 121. Popov, S.V.; Popova, G.Y.; Ovodova, R.G.; Ovodov, Y.S. Antiinflammatory activity of the pectic polysaccharide from comarum palustre. Fitoterapia 2005, 76, 281–287. [CrossRef] [PubMed] Int. J. Mol. Sci. 2017, 18, 709 21 of 23 122. Popov, S.V.; Ovodova, R.G.; Popova, G.Y.; Nikitina, I.R.; Ovodov, Y.S. Adhesion of human neuthrophils to fibronectin is inhibited by comuraman, pectin of marsh cinquefoil Comarum palustre L., and by its fragments. Biochemistry 2005, 70, 108–112. [PubMed] 123. Popov, S.V.; Ovodova, R.G.; Markov, P.A.; Nikitina, I.R.; Ovodov, Y.S. Protective effect of comaruman, a pectin of cinquefoil Comarum palustre L., on acetic acid-induced colitis in mice. Dig. Dis. Sci. 2006, 51, 1532–1537. [CrossRef] [PubMed] 124. Xie, G.; Schepetkin, I.A.; Siemsen, D.W.; Kirpotina, L.N.; Wiley, J.A.; Quinn, M.T. Fractionation and characterization of biologically-active polysaccharides from artemisia tripartita. Phytochemistry 2008, 69, 1359–1371. [CrossRef] [PubMed] 125. Surjushe, A.; Vasani, R.; Saple, D. Aloe vera: A short review. Indian J. Dermatol. 2008, 53, 163–166. [CrossRef] [PubMed] 126. Takahashi, M.; Kitamoto, D.; Asikin, Y.; Takara, K.; Wada, K. Liposomes encapsulating aloe vera leaf gel extract significantly enhance proliferation and collagen synthesis in human skin cell lines. J. Oleo Sci. 2009, 58, 643–650. [CrossRef] [PubMed] 127. Ghayempour, S.; Montazer, M.; Mahmoudi Rad, M. Simultaneous encapsulation and stabilization of Aloe vera extract on cotton fabric for wound dressing application. RSC Adv. 2016, 6, 111895–111902. [CrossRef] 128. Kitture, R.; Ghosh, S.; More, P.A.; Date, K.; Gaware, S.; Datar, S.; Chopade, B.A.; Kale, S.N. Curcumin-loaded, self-assembled aloe vera template for superior antioxidant activity and trans-membrane drug release. J. Nanosci. Nanotechnol. 2015, 15, 4039–4045. [CrossRef] [PubMed] 129. Esmaeili, A.; Ebrahimzadeh, M. Preparation of polyamide nanocapsules of Aloe vera L. Delivery with in vivo studies. AAPS Pharm. Sci. Tech. 2015, 16, 242–249. [CrossRef] [PubMed] 130. Deans, S.G.; Ritchie, G. Antibacterial properties of plant essential oils. Int. J. Food Microbiol. 1987, 5, 165–180. [CrossRef] 131. Kalemba, D.; Kunicka, A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 2003, 10, 813–829. [CrossRef] [PubMed] 132. Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [CrossRef] [PubMed] 133. Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [CrossRef] [PubMed] 134. El Asbahani, A.; Miladi, K.; Badri, W.; Sala, M.; Ait Addi, E.H.; Casabianca, H.; El Mousadik, A.; Hartmann, D.; Jilale, A.; Renaud, F.N.; et al. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 483, 220–243. [CrossRef] [PubMed] 135. Ajazuddin; Saraf, S. Applications of novel drug delivery system for herbal formulations. Fitoterapia 2010, 81, 680–689. [CrossRef] [PubMed] 136. Parris, N.; Cooke, P.H.; Hicks, K.B. Encapsulation of essential oils in zein nanospherical particles. J. Agric. Food Chem. 2005, 53, 4788–4792. [CrossRef] [PubMed] 137. Wu, Y.; Luo, Y.; Wang, Q. Antioxidant and antimicrobial properties of essential oils encapsulated in zein nanoparticles prepared by liquid–liquid dispersion method. LWT Food Sci. Technol. 2012, 48, 283–290. [CrossRef] 138. Pinho, E.; Grootveld, M.; Soares, G.; Henriques, M. Cyclodextrins as encapsulation agents for plant bioactive compounds. Carbohydr. Polym. 2014, 101, 121–135. [CrossRef] [PubMed] 139. Loftsson, T.; Brewster, M.E. Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J. Pharm. Sci. 1996, 85, 1017–1025. [CrossRef] [PubMed] 140. Ponce Cevallos, P.A.; Buera, M.P.; Elizalde, B.E. Encapsulation of cinnamon and thyme essential oils components (cinnamaldehyde and thymol) in β-cyclodextrin: Effect of interactions with water on complex stability. J. Food Eng. 2010, 99, 70–75. [CrossRef] 141. De Oliveira, E.F.; Paula, H.C.B.; de Paula, R.C.M. Alginate/cashew gum nanoparticles for essential oil encapsulation. Colloids Surf. B Biointerfaces 2014, 113, 146–151. [CrossRef] [PubMed] 142. Fleige, E.; Quadir, M.A.; Haag, R. Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: Concepts and applications. Adv. Drug Deliv. Rev. 2012, 64, 866–884. [CrossRef] [PubMed] 143. Bizzarro, V.; Carfagna, C.; Cerruti, P.; Marturano, V.; Ambrogi, V. Light-responsive polymer microcapsules as delivery systems for natural active agents. AIP Conf. Proc. 2016, 1736, 020078. Int. J. Mol. Sci. 2017, 18, 709 22 of 23 144. Marturano, V.; Cerruti, P.; Giamberini, M.; Tylkowski, B.; Ambrogi, V. Light-responsive polymer micro- and nano-capsules. Polymers 2017, 9, 8. [CrossRef] 145. Heras, B.D.L.; Hortelano, S. Molecular basis of the anti-inflammatory effects of terpenoids. Inflamm. Allergy Drug Targets 2009, 8, 28–39. [CrossRef] [PubMed] 146. Salminen, A.; Lehtonen, M.; Suuronen, T.; Kaarniranta, K.; Huuskonen, J. Terpenoids: Natural inhibitors of NF-κB signaling with anti-inflammatory and anticancer potential. Cell. Mol. Life Sci. 2008, 65, 2979–2999. [CrossRef] [PubMed] 147. Reddy, L.H.; Couvreur, P. Squalene: A natural triterpene for use in disease management and therapy. Adv. Drug Deliv. Rev. 2009, 61, 1412–1426. [CrossRef] [PubMed] 148. Spanova, M.; Daum, G. Squalene—Biochemistry, molecular biology, process biotechnology, and applications. Eur. J. Lipid Sci. Technol. 2011, 113, 1299–1320. [CrossRef] 149. Lacatusu, I.; Niculae, G.; Badea, N.; Stan, R.; Popa, O.; Oprea, O.; Meghea, A. Design of soft lipid nanocarriers based on bioactive vegetable oils with multiple health benefits. Chem. Eng. J. 2014, 246, 311–321. [CrossRef] 150. Ott, C.; Lacatusu, I.; Badea, G.; Grafu, I.A.; Istrati, D.; Babeanu, N.; Stan, R.; Badea, N.; Meghea, A. Exploitation of amaranth oil fractions enriched in squalene for dual delivery of hydrophilic and lipophilic actives. Ind. Crops Prod. 2015, 77, 342–352. [CrossRef] 151. Adamczak, M.; Kupiec, A.; Jarek, E.; Szczepanowicz, K.; Warszyński, P. Preparation of the squalene-based capsules by membrane emulsification method and polyelectrolyte multilayer adsorption. Colloids Surf. A Physicochem. Eng. Aspects 2014, 462, 147–152. [CrossRef] 152. Joscelyne, S.M.; Trägårdh, G. Membrane emulsification—A literature review. J. Membrane Sci. 2000, 169, 107–117. [CrossRef] 153. Das, B.; Yeger, H.; Baruchel, H.; Freedman, M.H.; Koren, G.; Baruchel, S. In vitro cytoprotective activity of squalene on a bone marrow versus neuroblastoma model of cisplatin-induced toxicity. Eur. J. Cancer 2003, 39, 2556–2565. [CrossRef] 154. Lin, Y.-K.; Al-Suwayeh, S.A.; Leu, Y.-L.; Shen, F.-M.; Fang, J.-Y. Squalene-containing nanostructured lipid carriers promote percutaneous absorption and hair follicle targeting of diphencyprone for treating alopecia areata. Pharm. Res. 2013, 30, 435–446. [CrossRef] [PubMed] 155. Gamze Kırkıl, M.D.; Muz, M.H.; Enver Sancaktar, M.D.; Dilara Kaman, M.D.; Şahin, K.; Küçük, Ö. The Effect of Lycopene Supplementation on Chronic Obstructive Lung Disease. Nobel Med. 2012, 8, 98–104. 156. Williams, E.J.; Baines, K.J.; Smart, J.M.; Gibson, P.G.; Wood, L.G. Rosuvastatin, lycopene and omega-3 fatty acids: A potential treatment for systemic inflammation in COPD; a pilot study. J. Nutr. Intermed. Metab. 2016, 5, 86–95. [CrossRef] 157. Butnariu, M.V.; Giuchici, C.V. The use of some nanoemulsions based on aqueous propolis and lycopene extract in the skin’s protective mechanisms against UVA radiation. J. Nanobiotechnol. 2011, 9, 3. [CrossRef] [PubMed] 158. Riangjanapatee, P.; Okonogi, S. Effect of surfactant on lycopene-loaded nanostructured lipid carriers. Drug. Discov. Ther. 2012, 6, 163–168. [CrossRef] [PubMed] 159. Nazemiyeh, E.; Eskandani, M.; Sheikhloie, H.; Nazemiyeh, H. Formulation and Physicochemical Characterization of Lycopene-Loaded Solid Lipid Nanoparticles. Adv. Pharm. Bull. 2016, 6, 235–241. [CrossRef] [PubMed] 160. Seo, E.Y.; Kim, M.H.; Kim, W.K.; Chang, M.J. Comparing the anti-inflammatory effect of nanoencapsulated lycopene and lycopene on RAW 264.7 macrophage cell line. J. Nutr. Health 2015, 48, 459–467. [CrossRef] 161. Serafini, M.R.; Menezes, P.P.; Costa, L.P.; Lima, C.M.; Quintans, L.J.; Cardoso, J.C.; Matos, J.R.; Soares-Sobrinho, J.L.; Grangeiro, S.; Nunes, P.S.; et al. Interaction of p-cymene with β-cyclodextrin. J. Thermal Anal. Calorim. 2012, 109, 951–955. [CrossRef] 162. De Santana, M.F.; Guimarães, A.G.; Chaves, D.O.; Silva, J.C.; Bonjardim, L.R.; Lucca Júnior, W.D.; Ferro, J.N.D.S.; Barreto, E.D.O.; Santos, F.E.D.; Soares, M.B.P.; et al. The anti-hyperalgesic and anti-inflammatory profiles of p-cymene: Evidence for the involvement of opioid system and cytokines. Pharm. Biol. 2015, 53, 1583–1590. [CrossRef] [PubMed] 163. De Souza Siqueira Quintans, J.; Menezes, P.P.; Santos, M.R.V.; Bonjardim, L.R.; Almeida, J.R.G.S.; Gelain, D.P.; Araújo, A.A.D.S.; Quintans-Júnior, L.J. Improvement of p-cymene antinociceptive and anti-inflammatory effects by inclusion in β-cyclodextrin. Phytomedicine 2013, 20, 436–440. [CrossRef] [PubMed] Int. J. Mol. Sci. 2017, 18, 709 23 of 23 164. Menezes, P.P.; Serafini, M.R.; Quintans-Júnior, L.J.; Silva, G.F.; Oliveira, J.F.; Carvalho, F.M.S.; Souza, J.C.C.; Matos, J.R.; Alves, P.B.; Matos, I.L.; et al. Inclusion complex of (−)-linalool and β-cyclodextrin. J. Thermal Anal. Calorim. 2014, 115, 2429–2437. [CrossRef] 165. Venâncio, A.M.; Marchioro, M.; Estavam, C.S.; Melo, M.S.; Santana, M.T.; Onofre, A.S.C.; Guimarães, A.G.; Oliveira, M.G.B.; Alves, P.B.; Pimentel, H.D.C.; et al. Ocimum basilicum leaf essential oil and (−)-linalool reduce orofacial nociception in rodents: A behavioral and electrophysiological approach. Rev. Bras. Farmacogn. 2011, 21, 1043–1051. [CrossRef] 166. Quintans-Júnior, L.J.; Barreto, R.S.S.; Menezes, P.P.; Almeida, J.R.G.S.; Viana, A.F.S.C.; Oliveira, R.C.M.; Oliveira, A.P.; Gelain, D.P.; de Lucca Júnior, W.; Araújo, A.A.S. B-cyclodextrin-complexed (−)-linalool produces antinociceptive effect superior to that of (−)-linalool in experimental pain protocols. Basic Clin. Pharmacol. Toxicol. 2013, 113, 167–172. [CrossRef] [PubMed] 167. Guimarães, A.G.; Oliveira, M.A.; Alves, R.D.S.; Menezes, P.D.P.; Serafini, M.R.; Araújo, A.A.D.S.; Bezerra, D.P.; Quintans Júnior, L.J. Encapsulation of carvacrol, a monoterpene present in the essential oil of oregano, with β-cyclodextrin, improves the pharmacological response on cancer pain experimental protocols. Chem. Biol. Interact. 2015, 227, 69–76. [CrossRef] [PubMed] 168. Lima, P.S.; Lucchese, A.M.; Araujo-Filho, H.G.; Menezes, P.P.; Araujo, A.A.; Quintans-Junior, L.J.; Quintans, J.S. Inclusion of terpenes in cyclodextrins: Preparation, characterization and pharmacological approaches. Carbohydr. Polym. 2016, 151, 965–987. [CrossRef] [PubMed] © 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).