* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Energy Band Diagrams - West Virginia University
Elementary particle wikipedia , lookup
Monte Carlo methods for electron transport wikipedia , lookup
Electric charge wikipedia , lookup
Nuclear structure wikipedia , lookup
Introduction to quantum mechanics wikipedia , lookup
Atomic nucleus wikipedia , lookup
Photoelectric effect wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
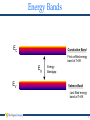
Overview of Silicon Device Physics Dr. David W. Graham West Virginia University Lane Department of Computer Science and Electrical Engineering 1 Silicon Silicon is the primary semiconductor used in VLSI systems Si has 14 Electrons Energy Bands (Shells) Valence Band Nucleus Silicon has 4 outer shell / valence electrons At T=0K, the highest energy band occupied by an electron is called the valence band. 2 Energy Bands } Increasing Electron Energy } Disallowed Energy States Allowed Energy States • Electrons try to occupy the lowest energy band possible • Not every energy level is a legal state for an electron to occupy • These legal states tend to arrange themselves in bands Energy Bands 3 Energy Bands EC Conduction Band First unfilled energy band at T=0K Eg EV Energy Bandgap Valence Band Last filled energy band at T=0K 4 Band Diagrams Increasing electron energy EC Eg EV Increasing voltage Band Diagram Representation Energy plotted as a function of position EC Conduction band Lowest energy state for a free electron EV Valence band Highest energy state for filled outer shells EG Band gap Difference in energy levels between EC and EV No electrons (e-) in the bandgap (only above EC or below EV) EG = 1.12eV in Silicon 5 Intrinsic Semiconductor Silicon has 4 outer shell / valence electrons Forms into a lattice structure to share electrons 6 Intrinsic Silicon The valence band is full, and no electrons are free to move about EC EV However, at temperatures above T=0K, thermal energy shakes an electron free 7 Semiconductor Properties For T > 0K Electron shaken free and can cause current to flow h+ e– • Generation – Creation of an electron (e-) and hole (h+) pair • h+ is simply a missing electron, which leaves an excess positive charge (due to an extra proton) • Recombination – if an e- and an h+ come in contact, they annihilate each other • Electrons and holes are called “carriers” because they are charged particles – when they move, they carry current • Therefore, semiconductors can conduct electricity for T > 0K … but not much current (at room temperature (300K), pure silicon has only 1 free electron per 3 trillion atoms) 8 Doping • Doping – Adding impurities to the silicon crystal lattice to increase the number of carriers • Add a small number of atoms to increase either the number of electrons or holes 9 Periodic Table Column 3 Elements have 3 electrons in the Valence Shell Column 4 Elements have 4 electrons in the Valence Shell Column 5 Elements have 5 electrons in the Valence Shell 10 Donors n-Type Material • • • • • • • • Donors Add atoms with 5 valence-band electrons ex. Phosphorous (P) “Dontates an extra e- that can freely travel around Leaves behind a positively charged nucleus (cannot move) Overall, the crystal is still electrically neutral Called “n-type” material (added negative carriers) ND = the concentration of donor atoms [atoms/cm3 or cm-3] ~1015-1020cm-3 e- is free to move about the crystal (Mobility mn ≈1350cm2/V) + 11 Donors n-Type Material • • • • • • • • Donors Add atoms with 5 valence-band electrons ex. Phosphorous (P) “Donates” an extra e- that can freely travel around Leaves behind a positively charged nucleus (cannot move) Overall, the crystal is still electrically neutral Called “n-type” material (added negative carriers) ND = the concentration of donor atoms [atoms/cm3 or cm-3] ~1015-1020cm-3 e- is free to move about the crystal (Mobility mn ≈1350cm2/V) n-Type Material + – + – + –+ – + + +– + – + – + – + – – + –+ + –+ – + +– – + – + – Shorthand Notation + Positively charged ion; immobile – Negatively charged e-; mobile; Called “majority carrier” + Positively charged h+; mobile; Called “minority carrier” 12 Acceptors Make p-Type Material • • • h+ – • • • • • Acceptors Add atoms with only 3 valenceband electrons ex. Boron (B) “Accepts” e– and provides extra h+ to freely travel around Leaves behind a negatively charged nucleus (cannot move) Overall, the crystal is still electrically neutral Called “p-type” silicon (added positive carriers) NA = the concentration of acceptor atoms [atoms/cm3 or cm-3] Movement of the hole requires breaking of a bond! (This is hard, so mobility is low, μp ≈ 500cm2/V) 13 Acceptors Make p-Type Material p-Type Material • – + – + + – +– + – – + + – – + – – + – + + – – – – + + – –+ + – + – + Shorthand Notation – Negatively charged ion; immobile + Positively charged h+; mobile; Called “majority carrier” – Negatively charged e-; mobile; Called “minority carrier” • • • • • • • Acceptors Add atoms with only 3 valenceband electrons ex. Boron (B) “Accepts” e– and provides extra h+ to freely travel around Leaves behind a negatively charged nucleus (cannot move) Overall, the crystal is still electrically neutral Called “p-type” silicon (added positive carriers) NA = the concentration of acceptor atoms [atoms/cm3 or cm-3] Movement of the hole requires breaking of a bond! (This is hard, so mobility is low, μp ≈ 500cm2/V) 14 The Fermi Function The Fermi Function • Probability distribution function (PDF) • The probability that an available state at an energy E will be occupied by an e- f(E) 1 f E 1 1 e E E f kT E Energy level of interest Ef Fermi level Halfway point Where f(E) = 0.5 k Boltzmann constant = 1.38×10-23 J/K = 8.617×10-5 eV/K T Absolute temperature (in Kelvins) 0.5 Ef E 15 Boltzmann Distribution If E E f kT f(E) Then f E e EE f kT 1 0.5 Boltzmann Distribution • Describes exponential decrease in the density of particles in thermal equilibrium with a potential gradient • Applies to all physical systems • Atmosphere Exponential distribution of gas molecules • Electronics Exponential distribution of electrons • Biology Exponential distribution of ions Ef ~Ef - 4kT E ~Ef + 4kT 16 Band Diagrams (Revisited) E EC Ef Eg EV Band Diagram Representation Energy plotted as a function of position EC Conduction band Lowest energy state for a free electron Electrons in the conduction band means current can flow EV Valence band Highest energy state for filled outer shells Holes in the valence band means current can flow Ef Fermi Level Shows the likely distribution of electrons EG Band gap Difference in energy levels between EC and EV No electrons (e-) in the bandgap (only above EC or below EV) EG = 1.12eV in Silicon 0.5 1 f(E) • Virtually all of the valence-band energy levels are filled with e• Virtually no e- in the conduction band 17 Effect of Doping on Fermi Level Ef is a function of the impurity-doping level n-Type Material E EC Ef EV 0.5 1 f(E) • High probability of a free e- in the conduction band • Moving Ef closer to EC (higher doping) increases the number of available majority carriers 18 Effect of Doping on Fermi Level Ef is a function of the impurity-doping level p-Type Material 1 f E E EC Ef EV 0.5 1 f(E) • Low probability of a free e- in the conduction band • High probability of h+ in the valence band • Moving Ef closer to EV (higher doping) increases the number of available majority carriers 19 Thermal Motion of Charged Particles • Applies to both electronic systems and biological systems • Look at drift and diffusion in silicon • Assume 1-D motion 20 Drift Drift → Movement of charged particles in response to an external field (typically an electric field) E-field applies force F = qE which accelerates the charged particle. However, the particle does not accelerate indefinitely because of collisions with the lattice (velocity saturation) Average velocity <vx> ≈ -µnEx electrons < vx > ≈ µpEx holes µn → electron mobility → empirical proportionality constant between E and velocity µp → hole mobility E µn ≈ 3µp 21 Drift Drift → Movement of charged particles in response to an external field (typically an electric field) E-field applies force F = qE which accelerates the charged particle. However, the particle does not accelerate indefinitely because of collisions with the lattice (velocity saturation) Average velocity <vx> ≈ -µnEx electrons < vx > ≈ -µpEx holes µn → electron mobility → empirical proportionality constant between E and velocity µp → hole mobility Current Density J n,drift mn qnE J p ,drift m p qpE q = 1.6×10-19 C, carrier density n = number of ep = number of h+ µn ≈ 3µp 22 Diffusion Diffusion → Motion of charged particles due to a concentration gradient • Charged particles move in random directions • Charged particles tend to move from areas of high concentration to areas of low concentration (entropy – Second Law of Thermodynamics) • Net effect is a current flow (carriers moving from areas of high concentration to areas of low concentration) dn x dx dp x qD p dx J n ,diff qDn J p ,diff q = 1.6×10-19 C, carrier density D = Diffusion coefficient n(x) = e- density at position x p(x) = h+ density at position x → The negative sign in Jp,diff is due to moving in the opposite direction from the concentration gradient → The positive sign from Jn,diff is because the negative from the ecancels out the negative from the concentration gradient 23 Einstein Relation Einstein Relation → Relates D and µ (they are not independent of each other) D kT m q UT = kT/q → Thermal voltage = 25.86mV at room temperature ≈ 25mV for quick hand approximations → Used in biological and silicon applications 24 p-n Junctions (Diodes) p-n Junctions (Diodes) • Fundamental semiconductor device • In every type of transistor • Useful circuit elements (one-way valve) • Light emitting diodes (LEDs) • Light sensors (imagers) 25 p-n Junctions (Diodes) p-type + – + – + – – + – + – + + – – + + – – + + – – + + – + – + – – + – + – + + – + – + – – + – + – + n-type – + – + – + + – + – + – – + – + – + – + – + – + – + – + – + + – + – + – – + – + – + + – + – + – Bring p-type and n-type material into contact 26 p-n Junctions (Diodes) p-type + – + – + – – + – + – + + – – + + – – + + – – + + – + – + – – + – + – + n-type + – + – + – – + – + – + – + – + – + + – + – + – – + – + – + – + – + – + – + – + – + + – + – + – – + – + – + + – + – + – Depletion Region • All the h+ from the p-type side and e- from the n-type side undergo diffusion → Move towards the opposite side (less concentration) • When the carriers get to the other side, they become minority carriers • Recombination → The minority carriers are quickly annihilated by the large number of majority carriers • All the carriers on both sides of the junction are depleted from the material leaving • Only charged, stationary particles (within a given region) • A net electric field This area is known as the depletion region (depleted of carriers) 27 Charge Density p-type + – + – + – Charge Density (x) – + – + – + + – – + + – – + + – – + + – + – + – – + – + – + n-type + – + – + – – + – + – + – + – + – + + – + – + – – + – + – + – + – + – + – + – + – + + – + – + – – + – + – + + – + – + – Depletion Region qND x -qNA The remaining stationary charged particles results in areas with a net charge 28 Electric Field Electric Field Charge Density (x) qND x -qNA E x • Areas with opposing charge densities creates an E-field • E-field is the integral of the charge density • Poisson’s Equation dE x dx ε is the permittivity of Silicon 29 Potential Electric Field Charge Density (x) qND x -qNA E x Potential • E-field sets up a potential difference • Potential is the negative of the integral of the E-field d E x dx bi x 30 Band Diagram qND x -qNA E Electric Field Charge Density (x) x Potential bi x Band Diagram EC • Line up the Fermi levels • Draw a smooth curve to connect them Ef EV 31 p-n Junction Band Diagram VA p n EC Ef EV p-type n-type 32 p-n Junction – No Applied Bias VA If VA = 0 p EC Ef EV n • Any e- or h+ that wanders into the depletion region will be swept to the other side via the E-field • Some e- and h+ have sufficient energy to diffuse across the depletion region • If no applied voltage Idrift = Idiff 33 p-n Junction – Reverse Biased VA If VA < 0 p Reverse Biased EC Ef EV n • Barrier is increased • No diffusion current occurs (not sufficient energy to cross the barrier) • Drift may still occur • Any generation that occurs inside the depletion region adds to the drift current • All current is drift current 34 p-n Junction – Forward Biased VA If VA < 0 p Forward Biased EC Ef EV n • Barrier is reduced, so more eand h+ may diffuse across • Increasing VA increases the eand h+ that have sufficient energy to cross the boundary in an exponential relationship (Boltzmann Distributions) →Exponential increase in diffusion current • Drift current remains the same 35 p-n Junction Diode I I0 e V A nUT 1 Diffusion Drift Combination of drift and generation UT kT → Thermal voltage = 25.86mV q 1 n 2 36 p-n Junction Diode I I0 e V A nUT I 0eVA nUT 1 I0 for VA > 0 for VA < 0 ln(I) I 1 q nU T nkT VA -I0 I I0 e VA V A nUT I eVA I0 nUT ln(I0) 1 1 I ln ln eVA nUT I0 ln I ln eVA nUT ln I 0 VA ln I ln I 0 nU T 37 Curve Fitting Exponential Data (In MATLAB) Curve Fitting Exponential Data (In MATLAB) I I 0 eVA nUT • Given I and V (vectors of data) • Use the MATLAB functions •polyfit – function to fit a polynomial (find the coefficients) •polyval – function to plot a polynomial with given coefficients and x values [A] = polyfit(V,log(I),1); % polyfit(independent_var,dependent_var,polynomial_order) % A(1) = slope % A(2) = intercept [I_fit] = polyval(A,V); % draws the curve-fit line 38