* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Introduction: Sources of electric current
Survey
Document related concepts
Transcript
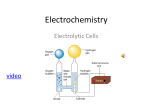
SOURCES OF ELECTRIC CURRENT There are several different devices that can supply the voltage necessary to generate an electric current. The two most common sources are generators and electrolytic cells. A Generators Generators use mechanical energy, such as water pouring through a dam or the motion of a turbine driven by steam, to produce electricity. The electric outlets on the walls of homes and other buildings, from which electricity to operate lights and appliances is drawn, are connected to giant generators located in electric power stations. Each outlet contains two terminals. The voltage between the terminals drives an electric current through the appliance that is plugged into the outlet. Power sources for generators can be steam from geothermal vents, or a nuclear reactor. Hydroelectricity would also fall under this category. B Electrolytic Cells Electrolytic cells use chemical energy to produce electricity. Chemical reactions within an electrolytic cell produce a potential difference between the cell’s terminals. An electric battery consists of a cell or group of cells connected together. C Other Sources Fuel Cells There are many sources of electric current other than generators and electrolytic cells. Fuel cells, for example, produce electricity through chemical reactions. Unlike electrolytic cells, however, fuel cells do not store chemicals and therefore must be constantly refilled. Certain sources of electric current operate on the principle that some metals hold onto their electrons more strongly than other metals do. Platinum, for example, holds its electrons less strongly than aluminum does. If a strip of platinum and a strip of aluminum are pressed together under the proper conditions, some electrons will flow from the platinum to the aluminum. As the aluminum gains electrons and becomes negative, the platinum loses electrons and becomes positive. This was observed in the lab activity we did. The strength with which a metal holds its electrons varies with temperature. If two strips of different metals are joined and the joint heated, electrons will pass from one strip to the other. Electricity produced directly by heating is called thermoelectricity. Photovoltaic Cells Some substances emit electrons when they are struck by light. Electricity produced in this way is called photoelectricity. When pressure is applied to certain crystals, a potential difference develops across them. Electricity thus produced is called piezoelectricity. Some microphones work on this principle.