* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download H. Hutten -
Remote ischemic conditioning wikipedia , lookup
Jatene procedure wikipedia , lookup
Heart failure wikipedia , lookup
Coronary artery disease wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Myocardial infarction wikipedia , lookup
Electrocardiography wikipedia , lookup
Ventricular fibrillation wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
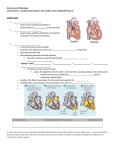
Ventricular intramyocardial electrograms and their expected potential for cardiac risk surveillance, telemonitoring and therapy management H. Hutten Graz University of Technology Abstract – Ventricular intramyocardial electrograms are recorded with electrodes directly from the heart either in intraventricular or epimyocardial position and may be acquired either from the spontaneously beating or from the paced heart. The morphology of these signals differs significantly from that of body surface ECG recordings. Although the morphology depends on individual impacts like the electrode position, the volume and anatomical shape of the heart and its changes during contraction, the dimensions of the fibrotic capsule forming around the electrode during the process of ingrowing etc, there is increasing evidence that intramyocardial electrograms have a high potential for cardiac risk surveillance, e.g. for arrhythmia detection, recognition of rejection events in transplanted hearts, and assessment of hemodynamic performance. Employing implants with telemetric capabilities renders possible permanent and even continuous cardiac telemonitoring. Furthermore the signals can be utilized for supporting therapy management, e.g. in patients with different kinds of cardiomyopathies. This paper shall demonstrate some preliminary results and discuss the expected potential. It will further discuss the problems of individual evaluation which requires approaches like personalized referencing based on similarity averaging and model-based signal interpretation. I. System or the increasing incidence of ventricular ectopic beats. Therefore the early recognition of such beginning episodes is of fundamental relevance. A suitable procedure for cardiac surveillance should allow quasi-continuous and in most cases permanent, i.e. lifelong, application. The used equipment should not constrain the patient in his daily routine activities and preferably not require special handling by the patient, but be forgettable if no risky situation is developing. There is increasing preliminary evidence that intramyocardial electrograms have the potential for efficient cardiac risk surveillance, especially if the system includes telemonitoring features, e.g. based on the Bluetooth and WLAN technology, cellular phone networks, intra- or internet technology or other global communication technologies which are already available, as well as advanced signal and information processing combined with powerful database systems. The most stringent constraint until now is the limited processing capacity of the microprocessors which are used in implants, usually 16-bit devices and in many cardiac pacemakers still 8-bit devices. If more tasks have to be accomplished, e.g. pacing, then the total load for the battery may be another limiting constraint. INTRODUCTION II. Diseases of the cardiovascular system are the major cause for morbidity, short term inability to work, lifelong invalidity, death and especially for health care expenditures in industrialized countries. Diagnostic terms like heart failure, cardiac dysfunction, myocardial infarction, cardiomyopathy, cardiac insufficiency, arrhythmia, and angina pectoris have become well-known to many people, primarily people older than 65 years. These diseases seem to be not longer confined to countries with a high living standard, but become an increasing problem also in countries with poor economy. Typical patients with a high risk potential for dramatic cardiac events are patients on dialysis, with kidney insufficiency, diabetes mellitus, high blood pressure, adiposity and certain lifestyle factors. Although many preventive programs have been proposed, the results until now are not really convincing. There is some evidence that programs aiming for changing the lifestyle do not find wide acceptance. Obviously individuals need the clear demonstration of their actual situation combined with a timely warning that a sudden event with high-risk potential may develop, e.g. the development of arrhythmia caused by a misbalance of the sympathetic and parasympathetic activity of the Autonomous Nervous METHODS A. Signal Acquisition Intramyocardial electrograms can be recorded with electrodes directly from the heart, either from the spontaneously beating or from the paced heart. These signals are usually utilized for the control of implanted cardiac pacemakers, e.g. the inhibition of pacing and the control of timing intervals. The intramyocardial electrograms have an amplitude of some millivolts and a frequency range between dc and about 150 Hz for the signals from the spontaneously beating heart and between dc and about 50 Hz for paced events. They are recorded usually in unipolar mode with the different electrode either in epimyocardial or intraventricular position and the pacemaker housing in some distance as the indifferent electrode. If the same electrode is employed for both pacing and recording of the Ventricular Evoked Response (VER), the electrode must have low polarization effect and short time constants for repolarization after pacing. These conditions are fairly well met by electrodes with artificially enlarged surfaces, e.g. by fractally coated or sputtered electrodes. Fig. 1 illustrates schematically the recording of intramyocardial electrograms with a dual-channel device, e.g. a DDD-mode pacemaker. Both channels may be used for pacing and/or recording. If electrode E1 is used for pacing, electrode E2 can be used to record the electrogram originating from electrode E1. Those signals aquired with the non-pacing electrode are frequently called VERX. The signal morphology of VERX signals ranges between those of VERs and those of spontaneous events, depending on the distance between the two electrodes E1 and E2. The analysis of VERX signals does not only allow to calculate the mean propagation velocity between electrode E1 (with proper consideration of the capture volume) and E2 by the delay between the stimulation in E1 and the appearance in E2, but may reveal histological information [1], e.g. about the fiber architecture between the two electrodes. However averaging is a proven method to improve the signalto-noise ratio and should preferably be used whenever applicable. Usually sequences of 1 minute are recorded and evaluated. Such sequences frequently contain not only VERs, but also spontaneous events and fusion beats of different degree. Fusion beats can show any signal morphology between that of spontaneous events and that of paced events. For that reason, a special procedure has been developed that uses similarity averaging instead of time averaging for ensemble averaging. It allows additionally to classify fusion beats by the degree of their deviation from the pure VER morphology. This renders possible some inference where the two excitation wavefronts have met between the pacing electrode and the origin of the spontaneous event. Fig. 3 shows a sequence of intramyocardial electrograms that presents paced events, spontaneous events and fusion beats as mixtures between these two types of different degree. Fig. 1: Schematic arrangement for recording intramyocardial electrograms with a dual-channel device. SVE: Spontaneous Ventricular Event, VER: Ventricular Evoked Response, VERX: Ventricular Evoked Response Crossed B. Signal Processing The signal morphology of intramyocardial electrograms as near-distant potential recordings is significantliy different from that of surface electrograms which are actually remote projections of the volume signal source in a certain plane, e.g. the usual bipolar EINTHOVEN leads in the frontal plane (fig. 2). Fig. 3: Sequence of intramyocardial electrograms that contains paced events (P), spontaneous events (S) and fusions of different degree (F). Signal processing whether aiming for RR-interval times series or for averaging requires the reliable and precise identification of a certain fiducial time point for each event. This is a rather simple task for paced events, however a challenge for spontaneous events and especially for fusion beats with their great variety in signal morphology. Hence, the detection algorithm must fulfill challenging requirements despite the limited performance of microprocessors in implants. The zero-crossing algorithm seems to be a promising candidate, but more advanced algorithms are required [2]. C. Signal Interpretation Fig. 2: Typical signal morphology of intramyocardial electrograms obtained from the spontaneously beating (a) and the paced heart (b) in comparison with body surface electrograms. Intramyocardial electrograms are less affected than surface recordings by such disturbances as the relative displacement of the heart against the electrode position caused by breathing, the changes in impedance and distance between the heart and the electrode due to different alveolar air filling, and muscle action potentials. Therefore evaluation of single events is possible. The signal morphology of intramyocardial electrograms significantly depends on the exact positioning of the electrode on or in the heart, on the volume and geometric shape of the heart as well as on the volume changes during contraction, and additionally about the growing of the fibrotic capsule around the electrode after implantation. For that reason the signal morphology of intramyocardial electrograms depends on individual factors, although the fundamental shape is comparable. If it is necessary to detect minor changes in the signal morphology, this can be reached by two methods: 1. The patient is used as “his own reference”. If signals can be acquired from the patient when he is in stable “normal” condition, they can subsequently be used to recognize deviations. Such deviations can be employed to identify their meaning for further examinations by statistical comparison with other symptoms. 2. A model-based approach is used to understand certain details of the signal morphology. Such models should be as realistic as possible, especially with regard to the geometry of the individual heart. If the whole signal is considered, i.e. both the depolarization and the repolarization phase, the model should also be capable to simulate the contraction. For that reason, 3D-images of the heart in enddiastolic and endsystolic state should be available and the position of the electrode (or of both electrodes) should be known as precisely as possible. Appropriate models for excitation generation and spreading as well as for contraction are now under development and may become available for individual matching in the near future [2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12]. D. Signal Transmission Remote cardiac risk surveillance requires the transmission of the relevant information which may be the whole sequence of intramyocardial electrograms or only an extracted risk parameter either to an extracorporeal station in order to warn the patient or to a central station which is in charge for the risk management. The transmission of the rather low-bandwidth electrograms (dc – 150 Hz) is not really a problem. The most serious problem until now is the limited battery power in the implant. For that reason transmission in available implants is restricted to the transmission either of an alarm signal only or to a “time window of interest” when the beginning of a risky situation is detected. In the CHARM project signal transmission has been accomplished after activation by inductive coupling which renders possible bi-directional transmission, i.e. to interrogate the actual pacemaker status [13]. Inductive coupling, however, will not be suitable for permanent monitoring. Fig. 4 shows a schematic illustration of the CHARM telemonitoring system. extracorporeal receiver station has to be limited with regard to the battery problem. But despite this constraint the mobility of the patient should not be restricted. At present only distances of about 2 m are acceptable. In future this problem may be solved by body-worn devices which are integrated into the clothing. These devices with both receiving and sending features may transmit the data via Bluetooth or wireless LAN technology to another near-by station that feeds the data into a global network system like the internet, the cellular phone network (e.g. Global System for Mobile Communications GSM or Universal Mobile Telecommunication System UMTS) or comparable technologies [14]. III. APPLICATIONS AND RESULTS A. Heart Transplant Rejection Heart transplant patients frequently suffer by rejection episodes. Preventive therapy is based on the application of immunosuppressive drugs with the consequence of enhanced risk for infections. Endomyocardial biopsy is the most widely accepted surveillance method which, however, is an invasive approach that can neither be standardized nor be applied for daily routine examinations and is rather expensive. In the CHARM-study (CHARM = Computerized Heart Acute Rejection Monitoring) it has been proven that evaluation of VERs allows reliable monitoring and shows good correlation with the results of endocardial biopsy. The patients are supplied with a pacemaker with broad-bandwidth telemetry for signal transmission to an extracorporeal data acquisition station. After acquisition the signals are transmitted via the internet to a central processing station in Graz [13, 15]. Fig. 5 illustrates a typical VER from a patient during normal cardiac condition, rejection and infection. Fig. 5: VERs from a heart transplant patient with the heart in normal condition, during a transient rejection episode and during an infection episode (pod = post-operative day). Fig. 4: Schematic illustration of the CHARM telemonitoring system [13] In any case, the transmission range from the implant to the B. Heart Rate Variability Heart rate variability is well accepted for risk stratification in patients. It is usually based on the determination of consecutive RR-intervals with subsequent evaluation either in the time or in the frequency mode. Details aiming for standardization have been published in 1996 by the Task Force of the European Society of Cardiology and the North American Society for Pacing and Electrophysiology [16]. The most urgent topic of this method is the assessment of the sympathetic and parasympathetic control of cardiovascular activity, primarily by the determination of the ratio of the spectral power density in the low and in the high frequency band. This allows also the assessment of the baroreflex sensitivity [17]. A shortcoming of this approach is that the evaluation requires more than 1 minute of steady state conditions, i.e. it can not be applied to short episodes of HRV fluctuations which seem to be relevant for continuous risk assessment. Therefore a special procedure (Multimodal Iterative Sinus Approximation) has been developed that considers appropriately all clinical experience as requested by the Task Force and allows to follow rapid HRV fluctuations [18, 19]. A simplified version of this algorithm can be implemented in a 16 bit-microprocessor in an implantable risk monitor [20]. Fig. 6 depicts the results obtained with this approach in the bimodal version for the evaluation of the fetal heart rate with this approach in the bimodal version. The rapid fluctuations of the spectral power density in the low frequency (LF) and in the high frequency (HF) band as well as the fluctuations of the 2 frequencies with the highest power density in these two frequency ranges demonstrate the superiority of this approach over the usual procedures based on Fast Fourier Transformation and Autoregressive Models. In this case the unborn baby was healthy, the fetal heart rate showed regular accelerations and decelerations [20]. baroreflex activity and thereby the Autonomous Nervous System with its feedback on the cardiac activity. This effect can be assessed globally with the parameters of the heart rate turbulence describing the dynamic transition of heart rate after an ectopic beat for about 20 s [21]. Another risk caused by ventricular ectopic beats may occur if they fall into the vulnerable period of the preceding beat. Fig. 7 illustrates the frequency of ventricular ectopic beats in a patient during hemodialysis [22]. The high rate of such ectopic beat has significant cardiovascular consequences. However, these patients are under high risk also at home, especially in the time after leaving the hospital and may benefit from continuous risk monitoring. Fig. 7: Time course of ventricular ectopic beats in a patient during hemodialysis. Each time segment corresponds to 5 minutes D. Hemodynamic Performance Intramyocardial electrograms are electrophysiological signals that represent the spatio-temporal spreading of the depolarizing and repolarizing wavefronts in the myocardium. Fig. 6: a. Records of fetal heart rate (upper part) and tocogram (lower part), b. HRV parameters LF Band, HF Band and characteristic frequencies obtained by application of the Bimodal Iterative Sinus Approximation [20] C. Ventricular Ectopic Beats Ventricular ectopic beats have some remarkable consequences. (1) The ventricular filling volume is reduced due to the shortened filling phase. (2) The atrial contraction is not preceding the ventricular contraction in proper time and hence does not effectively contribute to ventricular filling. (3) The spatio-temporal excitation spreading and, consequently the ventricular contraction sequence differs from that of a regular excitation originating from the Sinus Node and propagating along the specific conduction system. All these impacts result in a reduced left-ventricular stroke volume with a sudden decrease of the arterial blood pressure that stimulates the Fig. 8: Evaluation of the VER-parameter DAmp for 6 heart transplant patients in lying and supine position. The morphology of these signals, however, depends also on the geometric shape and volume of the heart, i.e. the enddiastolic and the endsystolic volume, and consequently monitors the stroke volume.. This dependence can in principle demonstrated using appropriate models. Until now, however, only very coarse models without sufficient individual matching have been made available. But in recent past that dependence could additionally be confirmed in patients by comparison with thermodilution and echocardiographic measurements and also by measurements during orthostatic tests and workload challenges [23, 24, 25]. It could even be demonstrated that parameters extracted from intramyocardial electrograms correlate with the NYHA classification and, hence, allow to monitor the efficiency of therapy management. Fig. 8 shows the results obtained from patients who underwent orthostatic tests. E. Therapy Management There is preliminary and challenging evidence that intramyocardial electrograms may be useful for supporting therapeutic procedures and therapy management. The fundamentals are based on the analysis and understanding of the signal morphology of fusion beats [26, 27]. Fusion beats can have different causes, but finally result if two depolarizing wavefronts meet with the consequence of mutual annihilation, i.e. none of the wavefronts proceeds beyond the meeting border line. These two wavefronts can originate from different sources, e.g. a spontaneous excitation from the Sinus Node meets with a paced event or with an ectopic beat, or the same excitation propagates on different pathways to the same syncytium, e.g. the excitation arrives in the ventricles via the HIS bundle and an accessory pathway. In pacemaker therapy the avoidance of fusion beats is of high relevance, both in order not to waste battery charge and also not to disturb the timing protocol. In the recent past the Ventricular Resynchronization Therapy has gained increased attention. It is based on multisite-pacing, e.g. biventricular pacing. The provoked fusion beats are followed by a changed spatio-temporal contraction as compared with regular excitation spreading. Interpretation of the intramyocardial electrograms representing fusion beats caused by multisite pacing may help to optimize the hemodynamic performance of the heart, e.g. by matching the delay between the two pacing pulses. In patients with Hypertrophic Obstructive Cardiomyopathy (HOCM) the contraction starts too early at the aortic outflow and thereby enlarges the outflow resistance before the ejection of blood by ventricular contraction begins. This enlarged outflow resistance causes hypertrophy of the ventricular muscle. The therapeutic concept utilizes a dual-chamber pacemaker that initiates ventricular contraction with sufficiently short atrio-ventricular interval (AVI) after sensing an atrial excitation in order to avoid the premature contraction at the aortic outflow. Fig. 9 illustrates the change in the morphology of the intramyocardial electrogram for different AVIs and the resulting impact on the Left Ventricular Outflow Tract Gradient (LVOTG) which is a measure for the aortic outflow resistance [28]. Fig. 9: Impact of different atrio-ventricular intervals (AVI) on the signal morphology of intramyocardial electrograms and the Left Ventricular Outflow Tract Gradient (LVOTG) in a patient Fig. 10 depicts intramyocardial electrograms acquired from two patients with dilative cardiomyopathy (i.e. candidates for heart transplantation) and from one patient after heart transplantation which show distinct differences in amplitude and morphology. These deviations may be caused primarily by differences in the stroke volume (or ejection fraction which usually in patients with dilative cardiomyopathy is much lower than in individuals with normal cardiac situation), but it may also be due to the enlargement of the heart silhouette and even the reduction in the thickness of the ventricular wall. Model based simulations have shown that these two variables can also affect the signal morphology. Fig. 10: Intramyocardial electrograms recorded from two patients with dilative cardiomyopathy and one patient after heart transplantation. F. Other applications Fig. 11 illustrates intramyocardial electrograms acquired from a heart transplant patient paced with different pacing rate. With higher pacing rate the deviation in the amplitude of the intramyocardial electrogram, especially in the period when contraction starts and the signal represents the enddiastolic volume, is significant and regular. This may be explained by a reduction in the enddiastolic filling volume. This finding may cause some speculations whether the effect can be utilized to develop a completely new generation of rate-adaptive cardiac pacemakers. Independent whether the adjustment of the stroke volume to a change in the hemodynamic requirement is transmitted primarily as inotropic information via the Autonomous Nervous System or by the FRANK-STARLING mechanism as in denervated hearts like in heart transplant patients, if it can be extracted, then it can be utilized for rate adaptation. Fig. 11: Recordings of intramyocardial electrograms acquired from a heart transplant patient during pacing with different pacing rates. IV. DISCUSSION Permanent and continuous monitoring of high-risk patients with cardiac failures as well as computer-assisted therapy management is an urgent problem in most industrialized countries and becoming an urgent problem also in other countries with poorer economy. Advanced information and communication technology is already available that renders possible the establishment of global networks for patient surveillance. The most difficult problem seems to be the identification and acquisition of the most suitable information, especially regarding the earliest detection of beginning lifethreatening events. Intramyocardial electrograms may open a new access to cardiac monitoring with regard to arrhythmia detection, recognition of dangerous ventricular ectopic beats, episodes of rejection in transplanted hearts, and other diseases resulting in cardiac insufficiency, but also with regard to the support of therapy management by drug application and adjustment of procedures like multisite pacing. A problem, however, that is not satisfactorily solved until now and requires more investigation is the understanding and interpretation of the signal morphology in order to extract reliable fiducial parameters and to “personalize” monitoring and therapy management. A promising approach is modelbased interpretation utilizing personalized 3D data sets [29]. ACKNOWLEDGMENT This work has in part been supported by the Austrian Science Fund in the Project No. P16965-N04 what is gratefully acknowledged. REFERENCES [1] [2] [3] [4] [5] [6] [7] [8] [9] Kraft, A., Schreier, G., Hutten, H., et al.: Assessment of Ventricular Excitation Propagation Velocity using Intramyocardial Electrograms and Electron Beam Tomography. Medical & Biological Engineering & Computing 37, Suppl. 2, Part II: 994 – 995 (1999) Stiegmaier, W., Hutten, H., Rauchegger, G.: Implementation of an Efficient R-Peak Detection Algorithm on a 16 bit Microprocessor for a Subcutaneously Implantable Heart Risk-Monitor. IFMBE Proc. 11(1): Proc. 3rd European Medical and Biological Engineering EMBEC’05 (ISSN 1727-1983): Paper No. 1611 (2005) LeGrice, I.J., Hunter, P.J., Small, B.H.: Laminar structure of the heart: a mathematical model. J. Physiol. 272: H2466 – H2476 (1997). LeGrice, I.J., Hunter, P.J., Young, A.A., Samll, B.H.: The architecture of the heart: a data-based model. Phil. Trans. R. Soc. A 359 : 1217 – 1232 (2001). Smith, N.P., Mulquiney P.J., Nash, M.P et al . : Mathematical modelling of the heart: cell to organs. Chaos, Solitons, Fractals 13: 1613 – 1621 (2001). Kohl, P., Noble, D., Hunter, P.J. (eds.): The integrated heart: modelling cardiac structure and function. Phil. Trans. R. Soc. A 359 (2001). Noble, D.: Modelling the heart: from genes to cells to the whole organ. Science 295: 1678 – 1682 (2002). Winslow, R.L., Helm, P., Baumgartner jr., W. et al.: Imaging-based integrative models of the heart: closing the loop between experiment and simulation. In: Bock, G., Goode, J.A. (eds.): “In silico” – Simulation of biological processes. Hoboken, John Wiley & Sons, Inc. : 129 – 140 (2002). Noble, D.: The heart cell in silic: success, failures and prospects. In: Bock, G., Goode, J.A. (eds.): “In silico” – Simulation of biological processes. Hoboken, John Wiley & Sons, Inc. : 182 – 1^94 (2002). [10] Sachse, F.B.: Computational Cardiology: Modeling of Anatomy, Electrophysiology, and Mechanics. LNCS 2966. Heidelberg, Springer Press (2004). [11] Seemann. G.: Modeling of electrophysiology and tension development in the human heart. PhD thesis, Institut fuer Biomedizinische Technik, Universitaetsverlag Karlsruhe ISBN 3-937300-66-X (2005). [12] L. Fritz, A. Prassl, H. Hutten et al.: Model-Based Analysis and Interpretation of Human Epicardial Electrograms using a Bidomain Slab Model. Proc. 3rd European Medical & Biological Engineering Conference EMBEC’05 2005:Paper No 2125 (2005) [13] H. Hutten, G. Schreier, P. Kastner et al.: CHARM – Computerized Heart Acute Rejection Monitoring. Biomedizinische Technik 41: 35 – 40 (1996). [14] Hutten, H., Richnovsky, D., Staber, G., Rauchegger, G.: Wireless ECG home monitoring system based on Bluetooth and GSM / GPRS. Electronics in Medicine 2004 (Cardiostim): St. Petersburg (Russia) 2004. [15] B. Grasser, B. Iberer, G, Schreier et al.: Computerized Heart Allograft Recipient Monitoring (CHARM) – a multicenter study. J. Heart Lung Transplant. 20: 214 (2001). [16] Task Force of the European Society of Cardiology and The North American Society of Pacing and Electrophysiology: Heart rate variability – Standards of measurement, physiological interpretation and clinical use. Eur. Heart J. 17: 354 – 381 (1996). [17] Fahn, B., Scharfetter, H., Wirnsberger, G., Hutten, H.: Spectral Analysis of Heart Rate Variability during Hemodialysis. Medical & Biological Engineering & Computing 37, Suppl. 2, Part II: 1280 – 1281 (1999) [18] Rauchegger, G., Hutten, H.: Kurzzeit-Herzratenvariabilitätsanalyse durch multimodale iterative Sinusapproximation. Biomedizinische Technik 48 (Ergänzungsband 1): 166 – 167 (2003). [19] Rauchegger, G., Hutten, H: Short-Time Heart-Rate-Variability Analysis with Recursive Multimodal Iterative Sine-Approximation. IFMBEProceedings, Vol. 6, 2004: MEDICON and HEALTH TELEMATICS 2004; CD-Ausgabe, ISSN: 1727-1983, ISBN: 88-7780-308-8 (2004). [20] Rauchegger, G.: Multimodale Iterative Sinusapproximation (MIS) – Eine dynamische Zeit-Frequenz-Transformation zur kardialen Risikostratifizierung. PhD-Thesis Graz University of Technology, (2004). [21] Schmidt, G., Malik, M., Barthel, P. et al.: Heart-rate turbulence after ventricular premature beats as a predictor of mortality after acute myocardial infarction. The Lancet 353: 1390 – 1396 (1999). [22] Andorfer, C., Hutten, H.: Kardiovaskuläres Risikomonitoring während der Hämodialyse durch EKG-Auswertung. Biomedizinische Technik 48 (Ergänzungsband 1): 494 – 495 (2003). [23] H. Hutten, P. Kastner, G. Schreier: Hemodynamic assessment by evaluation of intramyocardial electrograms. Proc. 20th Ann. Int. Conf. IEEE-EMBS Hongkong: 395 – 298 (1998). [24] Hribernigg, M., Ebner, E., Krätschmer, H., Hutten, H., Rauchegger, G.: Correlation Between Electrophysiologic and Hemodynamic Parameters of the Paced Heart. Proc. 2nd European Medical and Biological Engineering Conference EMBEC’02, Vol. 3 / 2002 in the IFMBE Proceedings Series, Part. I: 336 – 337 (2002). [25] Hribernigg, M., Ebner, E., Krätschmer, H., Hutten, H., Rauchegger, G.: Utilization of Intramyocardial Electrograms for Monitoring the Cardiac Performance in Accordance with the NYHA Classification. Proc. 2nd European Medical and Biological Engineering Conference EMBEC’02, Vol. 3 / 2002 in the IFMBE Proceedings Series, Part. I: 374 – 375 (2002). [26] H. Hutten, B. Tschapeller, L. Fritz: Some interesting Properties of Cardiac Fusion Beats. Proc. World Congress on Medical Physics and Biomedical Engineering 2006: 3303 – 3306 (2006). [27] L. Fritz, H. Hutten: Analysis of the Impact of Fusion Beats onto Epicardial Electrograms Based on a Bidomain Slab Model. Proc. World Congress on Medical Physics and Biomedical Engineering 2006: 3295 – 3298 (2006). [28] Schreier, G., Kastner, P., Hutten, H. et al.: Intramyocardial Electrogram Analysis for Hemodynamic Optimization in Patients Paced for Hypertrophic Obstructive Cardiomyopathy. Medical & Biological Engineering & Computing 37, Suppl. 2, Part I: 704 – 705 (1999) [29] Hutten, H., Tschapeller, B., Fritz, L., Rauchegger, G.: Model-Based Interpretation of Ventricular Evoked Responses. Proc. 17th Biennial Int. EURASIP Conf. BIOSIGNAL 2004. Jan J. Kozumplik, J., Provaznik, I. (eds.), Vutium Press (ISSN: 1211-412X), Brno University of Technology: 44 – 46 (2004).