* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Print - Circulation
Quantium Medical Cardiac Output wikipedia , lookup
Heart failure wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Myocardial infarction wikipedia , lookup
Electrocardiography wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Jatene procedure wikipedia , lookup
Heart arrhythmia wikipedia , lookup
Atrial fibrillation wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
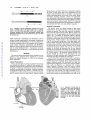
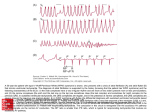
413 Ventricular Proarrhythmic Effects of Ventricular Cycle Length and Shock Strength in a Sheep Model of Transvenous Atrial Defibrillation Gregory M. Ayers, MD, PhD; Clif A. Alferness, BSEE; Marina Ilina, PhD; Darrell 0. Wagner, ASET; William A. Sirokman, BS; John M. Adams, BSEE; Jerry C. Griffin, MD Downloaded from http://circ.ahajournals.org/ by guest on June 14, 2017 Background Synchronized cardioversion is generally accepted as safe for the treatment of ventricular tachycardia and atrial fibrillation when shocks are synchronized to the R wave and delivered transthoracically. However, others have shown that during attempted transvenous cardioversion of rapid ventricular tachycardia, ventricular fibrillation (VF) may be induced. It was our objective to evaluate conditions (short and irregular cycle lengths [CL]) under which VF might be induced during synchronized electrical conversion of atrial fibrillation with transvenous electrodes. Methods and Results In 16 sheep (weight, 62±7.8 kg), atrial defibrillation thresholds (ADFT) were determined for a 3-ms/ 3-ms biphasic shock delivered between two catheters each having 6-cm coil electrodes, one in the great cardiac vein under the left atrial appendage and one in the right atrial appendage along the anterolateral atrioventricular groove. A hexapolar mapping catheter was positioned in the right ventricular apex for shock synchronization. In 8 sheep (group A), a shock intensity 20 V less than the ADFT was used for testing, and in the remaining 8 sheep (group B), a shock intensity of twice ADFT was used. With a modified extrastimulus technique, a basic train of eight stimuli alone (part 1) and with single (part 2) and double (part 3) extrastimuli were applied to right ventricular plunge electrodes. Atrial defibrillation shocks were delivered synchronized to the last depolarization. In part 4, shocks were delivered during atrial fibrillation. The preced- ing CL was evaluated over a range of 150 to 1000 milliseconds. Shocks were also delayed 2, 20, 50, and 100 milliseconds after the last depolarization from the stimulus (parts 1 through 3) or intrinsic depolarization (part 4). The mean ADFT for group A was 127±48 V, 0.71±0.60 J and for group B, 136+37 V, 0.79±0.42 J (NS, P>.15). Of 1870 shocks delivered, 11 episodes of VF were induced. Group A had no episodes of VF in part 1, two episodes of VF in part 2 (CL, 240 and 230 milliseconds with 2-millisecond delay), and one episode each in parts 3 (CL, 280 milliseconds with 2-millisecond delay) and 4 (CL, 240 milliseconds with 100-millisecond delay). Group B had two episodes in part 1 (CL, 250 and 300 milliseconds with 20-millisecond delay), three episodes in part 2 (CL, 230, 230, and 250 milliseconds with 2-millisecond delay), and one episode each in parts 3 (CL, 260 milliseconds with 2-millisecond delay) and 4 (198 milliseconds with 100-millisecond delay). No episodes of VF were induced for shocks delivered after a CL >300 milliseconds. Conclusions Synchronized transvenous atrial defibrillation shocks delivered on beats with a short preceding ventricular cycle length (<300 milliseconds) are associated with a significantly increased risk of initiation of VF. To decrease the risk of ventricular proarrhythmia, short CLs should be avoided. (Circulation. 1994;89:413-422.) Key Words * atrial fibrillation * ventricles * fibrillation * defibrillation * heart rate t has been recognized since the early 1960s that electrical reversion of cardiac arrhythmias is effec_ tive.12 Equally well known are the ventricular arrhythmias, tachycardia and fibrillation, associated with cardioversion of atrial arrhythmias.2-5 These proarrhythmic side effects are attributed to vulnerable-period Although the timing relation and duration are somewhat disputed, shocks delivered during some portion of the T wave consistently result in ventricular fibrillation.3-5 The atrial vulnerable period has also been documented. During normal sinus rhythm, the atrial vulnerable period is on the downslope of the R wave of the surface ECG.' With the advent of the R-wave synchronized defibrillator, the risk of ventricular arrhythmia induction during cardioversion of atrial fibrillation was lessened. Recent data have shown that atrial fibrillation is associated with a 2.2% to 2.5% risk of arrhythmic death without concomitant antiarrhythmic therapy6'7 and 5% in patients treated with antiarrhythmic agents.6 It is therefore apparent that cardioversion of atrial fibrillation to sinus rhythm is of significant importance and that all efforts must be expended to minimize risk. Although isolated empirical observations have been made with respect to the induction of ventricular fibrillation during synchronized, transvenous cardioversion of atrial fibrillation,8-10 no study has been undertaken to systematically model and evaluate the conditions under stimulation. The existence of two vulnerable periods has been well documented for the heart.1-5 During these periods, either the atria or ventricles are susceptible to fibrillation. The vulnerable period is directly related to the relative refractory period during which the heart is susceptible to fibrillation induction by a stimulus. For ventricular myocardium, this period is near the apex of the T wave. Received June 11, 1993; revision accepted August 3, 1993. From InControl, Inc, Redmond, Wash (G.M.A., C.A.A., M.I., D.O.W., W.A.S., J.M.A., J.C.G.) and University of California San Francisco (J.C.G.). Correspondence to Gregory M. Ayers, MD, PhD, Senior Scientist, InControl, Inc, 6675 185th Ave NE, Suite 100, Redmond, WA 98052. 414 Circulation Vol 89, No 1 January 1994 4 Lk -- 6CM. - ~ ~ ~ _ . m ~ A~:_: aW :E h p RPLt * UW X (a) |4 - 6CM. - - (b) FIG 1. Diagrams of the two defibrillation catheters used in this study. a, Catheter used in the great cardiac vein; b, catheter used in the right atrial appendage. Both were modified 6F hexapolar catheters with a 6-cm silver-plated stainless steel electrode coil attached to the sixth electrode ring (a) or the distal tip electrode (b). Downloaded from http://circ.ahajournals.org/ by guest on June 14, 2017 which ventricular arrhythmias and fibrillation can be induced during synchronized, low-energy, transvenous cardioversion of atrial fibrillation. It was our objective to evaluate conditions (short and irregular cycle lengths) under which ventricular arrhythmias and fibrillation might be induced during synchronized electrical conversion of atrial fibrillation with a transvenous electrode system that has been shown"": 2 to be effective for atrial defibrillation. Methods This study involving experimental animals conformed to the positions of the American Heart Association on research animal use adopted November 11, 1984, by the American Heart Association. Animal Preparation Sixteen sheep (weight, 62±7.8 kg) of random breed and sex were sedated with xylazine 0.2 mg/kg IM and morphine 10.0 mg/kg IM. Anesthesia was induced with sodium pentobarbital 25 mg/kg IV. Additional pentobarbital was used as needed to maintain a constant plane of anesthesia. Additional morphine 5.0 mg/kg IM was given every 4 hours. A femoral artery was cannulated to monitor arterial blood pressure and the femoral vein for venous access for drug delivery. Femoral arterial blood pressure and lead II ECG were monitored throughout the study. Arterial blood gases were determined every 30 minutes, with adjustments made to ventilation or intravenous sodium bicarbonate given on the basis of the results. Serum electrolytes, including potassium, calcium, and magnesium, were determined from blood drawn each hour, with supplemental electrolytes added to the intravenous fluids to maintain normal serum levels. Sodium heparin 10 000 U IV was given after initial surgical preparation. Succinyleholine 0.6 mg/kg IV was administered before each shock delivery sequence. Surgical Preparation The thoracic cavity was opened through a right lateral thoracotomy at the fourth interspace with the pericardium isolated and opened. The heart was cradled in the pericardium. Three 9F catheter sheaths (USCI, Billerica, Mass) were inserted, two into the right jugular vein and one into the left jugular vein. A modified 6F hexapolar catheter (Elecath, Rahway, NJ) with a 6-cm silver-plated stainless steel electrode coil (Renton Coil Spring, Renton, Wash, and ASKO Selective Plating, Seattle, Wash) attached to the sixth electrode ring (Fig la) was inserted through one of the right jugular vein sheaths, passed through the coronary sinus, and positioned in the great cardiac vein (Fig 2). This catheter was advanced until the catheter tip was in the anterior coronary vein. A second modified 6F hexapolar catheter with a 6-cm coil electrode attached to the distal tip electrode (Fig lb) was inserted through the second jugular vein sheath on the right side and advanced so that the tip was in the right atrial appendage (Fig 2). A third catheter, a 6F hexapolar mapping catheter, was inserted through the sheath in the left jugular vein and advanced to the right ventricular apex. Plunge electrodes were placed into the right ventricular free wall (Fig 2). Lead II surface ECG, systemic blood pressure, and bipolar electrograms were recorded with a custom, isolated interface/ amplifier (InControl, Redmond, Wash), digital storage oscilloscope (Tektronix model 2232, Beaverton, Ore) and Teac model RD130/T digital tape recorder (Teac America, Montebello, Calif) and digitized on a Macintosh TTfx computer (Apple Computer, Cupertino, Calif) with custom LABVIEW software (National Instruments, Austin, Tex). Bipolar electrograms were recorded from the great cardiac vein coil electrode to right atrial coil electrode and right ventricular tip to a proximal right ventricular electrode. Digital recordings of both normal sinus rhythm and atrial fibrillation were made at the FIG 2. Diagrams of frontal and lateral views of the heart showing the locations of the three 6F catheter locations, great cardiac vein (CS/ GCV), right atrial appendage (RA), and right ventricular apex (RV). Also shown are the two plunge electrodes positioned on the right ventricular free wall. 2) RV Plunge Electrodes Ayers et al Rate Effect on Atrial Defibrillation Proarrhythmia 415 |3 ms.| Vif V 2i V2j = -Vif - W v3ms. 1 1 FIG 3. Diagram of the defibrillation shock waveform used in this study. It is a single-capacitor 90-,uF biphasic waveform with both phases having a 3-millisecond duration. Downloaded from http://circ.ahajournals.org/ by guest on June 14, 2017 beginning of each study. Critical events were marked and later retrieved from digital tape for analysis of electrograms. After completion of the study, the animals were killed with a premixed solution of pentobarbital, 6 grains/mL (Anthony Products, Arcadia, Calif). Atrial Defibrillation Threshold Determination After surgical preparation, atrial defibrillation thresholds were determined for a 3-ms/3-ms single-capacitor biphasic shock (Fig 3). Atrial fibrillation was electrically induced with 10-mA, 1-millisecond pulses at a frequency of 60 Hz with epicardial wires attached to the right atrium. Before the first defibrillation attempt, atrial fibrillation was permitted to sustain for 5 minutes to demonstrate adequate stability. If atrial fibrillation spontaneously terminated in less than 5 minutes, t3-methacholine, 50 ,ug/250 mL normal saline, was applied to the atrial epicardium in a continuous infusion.13 Before each defibrillation shock, atrial fibrillation was induced and permitted to continue for 30 seconds. For every atrial defibrillation attempt, the great cardiac vein coil electrode served as the cathode and the right atrial coil electrode as the anode, with respect to the first phase of the shock. For each shock, a customized LABVIEW software package running on a Macintosh IIfx computer controlled the shock delivery from a custom defibrillator (InControl) and acquired the delivered voltage and current waveforms. From the acquired shock data, the software calculated the delivered energy and shock impedance. All shocks were synchronized to the V wave sensed from the right ventricular catheter. Atrial defibrillation thresholds were determined with the algorithm shown in Fig 4. Starting at a peak leading-edge voltage of 80 V, atrial defibrillation shocks were delivered to the catheter electrode system. The philosophy of this threshold method was to determine the lowest-intensity shock that would defibrillate the atria and then determine the percent successful defibrillation at this intensity. If the shock failed to defibrillate, the shock intensity was increased by 10 V. Once the shock was successful, atrial fibrillation was induced, and 20 shocks were delivered at this intensity to determine the percent success. After each successful shock, atrial fibrillation was initiated, and 30 seconds was allowed to elapse before the next shock was delivered. If the percent success was < 10%, the percent success was determined at a shock voltage 10 V higher than just tested. If the percent success was >90%, the percent success was determined at an intensity 10 V less than the intensity just tested. Thus, the threshold was considered valid if the resulting percent success was between 10% and 90%. Ventricular Vulnerability Testing The 16 sheep in this study were divided into two groups for study of the effect of shock strength on the likelihood of Yes |ADFT | FIG 4. Algorithm used to define atrial defibrillation thresholds in this study. ventricular arrhythmia induction. Group A received subthreshold atrial defibrillation shocks (20 V less than threshold), and group B received suprathreshold shocks (twice threshold). Both groups underwent a four-part ventricular vulnerability testing protocol. Pacing studies, as with the defibrillation threshold testing, were performed with custom LABVIEW software running on a Macintosh IIfx platform. The software permitted standard programmed stimulation as well as combined pacing and defibrillation control (Fig 5). Pacing threshold current was determined for the bipolar right ventricular plunge electrodes with a 1-millisecond square wave at a cycle length of 300 milliseconds. When a cycle length of 300 milliseconds did not consistently capture the ventricles because of the rate, the cycle length was increased in steps of 50 milliseconds until consistent capture of a train of eight was achieved. Intensity of twice pacing threshold current was used for all stimuli in the remainder of the study. A modified extrastimulus technique was used for the first three parts (parts 1 through 3) of the protocol. These three parts were performed in random order. In part 4, shocks were delivered during atrial fibrillation. Shocks were delivered synchronously to depolarizations sensed from the right ventricular apex catheter in all parts. Shock delay at each testing cycle length started with 100 milliseconds and was decreased to 50, 20, and 2 milliseconds. Incidence of postshock sustained ventricular arrhythmias was documented, and the digital tape recording of electrograms was marked for later retrieval. 416 Circulation Vol 89, No 1 January 1994 FIG 5. Diagram of the interconnections between the various devices used in this study and the animal. RV indicates right ventricular apex recording catheter; GCV, great cardiac vein defibrillation catheter; and RA, right atrial appendage defibrillation catheter. Enable and Si, S2, S3 are the computer control lines for the custom defibrillator and programmable stimulator, respectively. Downloaded from http://circ.ahajournals.org/ by guest on June 14, 2017 delivered 100 milliseconds after the sensed depolarization of the last S, from the right ventricular apex catheter. If ventricular fibrillation was not induced, the sequence of S, stimuli and shock was repeated, but the delay was reduced to 50, then 20, and last, 2 milliseconds. If ventricular fibrillation was not induced, the cycle length of the train was decreased by 50 Part 1 This part of the protocol modeled rapid but regular cycle lengths (Fig 6a). A train of eight stimuli (S,) was delivered to the right ventricular plunge electrodes starting at a cycle length of 600 milliseconds. An atrial defibrillation shock was Sl Si s1 (a) A. 1---r do L----a EI S1 - Si 51 £I Sli S1 SHOCK r_ fSe -e# 51 n C dA.. 51 sI 51 S1 S2 SHOCK (b) it i 1- Si 51 li ----i 51 ---------------------- ---L m li 51 51 CC - 51 1l S2 S3 VSHOCK (c) a ------- a a -- ---- ------- -1- -1 --------------------- L%- .<z Ventricular depolarizations in AF 1v V \v, (d) ' 10r 5 2.170 i2so tt 32 40 30 V ISHOCK 36 a I~~~~ 350-400 msec window FIG 6. Schematic of each part of the protocol. A basic train of eight, part 1 (a), single, part 2 (b), and double, part 3 (c) extrastimuli were applied with a shock delivered after the sensed depolarization from the last stimulus. The shock was delayed 100 milliseconds after this depolarization. The shock delay was then decreased to 50, 20, and then 2 milliseconds. The pacing intervals, S,-S, in part 1, S1-S2 in part 2, and S2-S3 in part 3, were decremented and all shock delays retested. In part 4 (d), ventricular depolarizations were sensed and cycle lengths calculated and compared with a 50-millisecond shock window. When a preceding cycle length was within the window, a shock was delivered delayed from the ventricular depolarization. This delay was then reduced from 100 to 50, 20, and then 2 milliseconds. Ayers et al Rate Effect on Atrial Defibrillation Proarrhythmia milliseconds and the sequence of delays repeated. This continued either until ventricular fibrillation was induced or until the cycle length reached the effective refractory period so that all eight S, stimuli were not capturing the ventricle. Part 2 This part of the protocol modeled rapid and regular cycle lengths followed by a premature complex (Fig 6b). A train of eight stimuli (S,) at the shortest cycle length found during threshold testing to produce consistent capture (300 milliseconds, seven sheep; 350 milliseconds, eight sheep; and 400 milliseconds, one sheep) was delivered to the plunge electrodes, followed by a premature stimulus (S2). The S1-S2 interval started at 290 milliseconds, with the shock delayed 100 milliseconds from the sensed depolarization. As in part 1, the delay was reduced to 50, then 20, then 2 milliseconds at a constant S1-S2 interval, then the S1-S2 interval was decreased in 10-millisecond steps and the sequence of delays repeated at Downloaded from http://circ.ahajournals.org/ by guest on June 14, 2017 each S1-S2 interval either until ventricular fibrillation was induced or until the S2 failed to capture the ventricle. An epinephrine drip (2 mg/250 mL normal saline) was then infused at a rate resulting in a mean blood pressure rise to twice the preinfusion baseline value to further shorten ventricular refractoriness. The delay scanning and 10-millisecond S1-S2 interval decreases were resumed until the S2 again failed to initiate a depolarization or until ventricular fibrillation was induced. Part 3 This part of the protocol modeled a series of short intervals followed by a long interval, at least twice the cycle length of the basic train, then a short interval with a shock after the last depolarization (Fig 6c). A basic train of eight stimuli (S,), with a cycle length equal to the train used in part 2, was delivered 10 times to determine the time from end of the train to the first intrinsic ventricular beat (V2). The minimum S,-V2 interval was then compared with the drive train S,-S, interval, and if it was found to be at least twice the S,-S, interval, this part of the study was performed. A premature stimulus (S2) was added to the basic train of eight with an S1-S2 interval equal to 50 milliseconds less than the minimum S,-V2 interval. A second premature stimulus (S3) was added to the pacing train at an initial S2-S3 interval of 290 milliseconds. Shocks were again delivered delayed by 100, then 50, 20, and 2 milliseconds from the last depolarization from the pacing stimuli. The S2-S3 interval was decreased by 10 milliseconds, testing each delay in decremental manner either until S3 failed to capture the ventricle or until ventricular fibrillation was induced. Part 4 In this part of the protocol, shocks were delivered during electrically induced atrial fibrillation. The defibrillation control software for shock delivery selected only ventricular depolarizations whose preceding cycle lengths fell into a 50-millisecond window (Fig 6d). We tested 50-millisecond windows from 150 to 200 milliseconds up to 950 to 1000 milliseconds. Atrial fibrillation was induced and permitted to sustain for 5 minutes. The customized defibrillation control software was configured to deliver a 100-millisecond delayed shock on the first preceding cycle length that fell into a window of 950 to 1000 milliseconds. The other three delays were tested, 50, then 20, then 2 milliseconds, for the same cycle length window. The window was then shifted down in steps of 50 milliseconds, with all delays tested at each window of cycle lengths. A time limit of 2 minutes was placed on the length of time the defibrillator would wait for a given window. If no intervals were found during this 2-minute period, the window was shifted by 50 milliseconds and testing was resumed. If the time-out period was exceeded because of a lack of adequately short cycle 417 TABLE 1. Heart and Body Weight, Threshold Data and Stimulation Data, and S, Cycle Length From the Animals in This Study Group A Group B Body weight, kg 58.4+4.9 64.6±9.1 Heart weight, g 361.9±57.1 399.3±120.9 ADFT, V 127.5±48.0 136.3±36.6 ADFT, J 0.71±0.60 0.79±0.42 Percent success at ADFT 52.0±31.3 48.1±29.3 S1 cycle length, msec 319±26 343±32 ADFT indicates atrial defibrillation threshold. With the exception of S, cycle length, there were no differences between groups A and B (P>.1). Values are mean±SD. lengths, an epinephrine infusion (2 mg/250 mL normal saline) was started and delivered at a rate to double the mean preinfusion blood pressure, and testing was resumed. Testing continued until the time period at the window of 150 to 200 milliseconds expired or at a window and delay that generated ventricular fibrillation. If ventricular fibrillation was induced, the cycle length range was documented and the tape recording of the electrograms was marked electrically. After the study, electrograms were replayed to a Gould TA4000 paper recorder (Gould Inc, Cleveland, Ohio), and the preceding cycle length was measured for the shocks that resulted in ventricular fibrillation. Statistical Analysis of Data All statistical analyses were performed using JMP software (SAS Institute, Cary, NC) running on a Macintosh Quadra 700 platform (Apple Computer). Continuous numerical data requiring comparison were subject to ANOVA for comparing the effect of group differences. These data included body and heart weights, atrial defibrillation thresholds, and stimulus parameters. Comparisons in the episodes of ventricular fibrillation induced between groups and parts were made by x2 tests. Differences were considered significant at values of P<.05. Results The mean atrial defibrillation threshold voltage and energy are shown in Table 1. Also shown are the mean S, interval used for the stimulus train in parts 2 and 3 of the protocol. There was no significant difference in the animal characteristic or threshold data when groups A and B were compared, although the mean S1 for group B was longer (P<.05). Not including the shocks used to determine threshold, 1870 test shocks were delivered in this study. Eleven episodes (0.59%) of ventricular fibrillation were induced in 8 of the 16 sheep with these shocks, 4 sheep in group A and 4 in group B. Table 2 details the episodes of ventricular fibrillation induced. All of the episodes of ventricular fibrillation were induced with a preceding cycle length 5300 milliseconds. In part 1, two episodes of ventricular fibrillation were induced out of the 348 shocks delivered (0.57%). Both episodes were induced for animals in group B, twice atrial defibrillation threshold, although not statistically significantly different from group A by a x2 test (P> .15). Both episodes were induced with a right ventricular depolarization-to-shock delay of 20 milliseconds. An example of one ventricular fibrillation induction in this part of the study is shown in Fig 7A. 418 Circulation Vol 89, No 1 January 1994 TABLE 2. Episodes of Ventricular Fibrillation, by Sheep and Protocol Part, With Corresponding Preceding Ventricular Cycle Length and Shock Delay Protocol Part Sheep Group A 1 2 3 4 5 6 7 8 1 2 3 4 S1-S1, Delay, ms S1-S2, Delay, ms S1-S2, 52-S3, Delay, me Cycle Length, Delay, ms n n n n n n n n 240,2 x 740,280,2 x n n x x n n n n n n n n 240,100 n n n n n n n 198,100 x n n n n n n n n n n n 230,2 n n Group B Downloaded from http://circ.ahajournals.org/ by guest on June 14, 2017 9 10 11 12 13 14 15 16 n x 300,20 250,2 n n n n n n 250,20 230,2 550,260,2 x n 230,2 Eleven episodes of ventricular fibrillation were induced by the ventricular vulnerability protocol in this study. The letter n indicates lack of induction of ventricular fibrillation. In part 3, for seven sheep the intrinsic escape interval was too rapid and therefore did not permit the delivery of a long S2-S3 interval, so this part was omitted, denoted by the letter x. In part 2, five episodes of ventricular fibrillation were induced out of 568 shocks delivered (0.88%). There were three for group B and two for group A (NS, P>.15). All five episodes were induced with a right ventricular depolarization-to-shock delay of 2 milliseconds and preceding cycle lengths .250 milliseconds. An example of one ventricular fibrillation induction in this part of the study is shown in Fig 7B. During part 3, two episodes of ventricular fibrillation were induced out of 238 shocks delivered (0.84%). Each group had one sheep with an episode of ventricular fibrillation induced during this part of the protocol. For one sheep, the S1-S2 interval was 740 milliseconds followed by an S2-S3 of 280 milliseconds, and for the other sheep, the S1-S2 interval was 550 milliseconds followed by an S2-S3 interval of 260 milliseconds. Both occurred with a right ventricular depolarization-toshock delay of 2 milliseconds. An example of one ventricular fibrillation induction in this part of the study is shown in Fig 7C. In part 4, two episodes of ventricular fibrillation were induced out of 716 shocks (0.28%). There was one episode for each group. Because the preceding cycle length was not paced in this part but rather was selected by the defibrillator-computer system to fall within a preselected range, the exact preceding interval was measured after completion of the study from the taperecorded electrograms. For one sheep, the preceding cycle length range was 150 to 200 milliseconds, with a measured cycle length of 198 milliseconds, and for the other sheep, the preceding cycle length range was 200 to 250 milliseconds, with a measured preceding cycle length of 240 milliseconds. Both episodes of ventricular fibrillation were initiated with shock delays of 100 milliseconds. An example of one ventricular fibrillation induction in this part of the study is shown in Fig 7D. To analyze the dependence of vulnerability on cycle lengths, an arbitrary cycle length of 300-millisecond cutoff was used because it was the longest cycle length preceding a shock that induced ventricular fibrillation. There were 11 episodes of induced ventricular fibrillation out of 975 shocks with a preceding cycle length s300 milliseconds (1.1%) and no episodes of ventricular fibrillation out of 895 shocks with a preceding cycle length >300 milliseconds. The episodes of ventricular fibrillation were compared on a sheep-by-sheep basis by a McNemar's test and found to be significantly different at P<.05. A paired t test comparing the number of shocks delivered to individual sheep showed that the number of shocks delivered on intervals <300 milliseconds were not significantly different from the number of shocks delivered on intervals >300 milliseconds, P>.05. Discussion New Findings of This Study In this study, 11 episodes of ventricular fibrillation were induced during the delivery of 1870 atrial defibrillation shocks. We have shown that shocks delivered with Ayers et al Rate Effect on Atrial Defibrillation Proarrhythmia si si si Si .S Sl SlSl S1 :mV Si Si S1 SI S 419 12 Surface: ECG Surface ECG i 1 mY Atrium IlmV Atrium A V lAI sticl 1secon7d* shock 1 second B A Surfecb S 1 SI SISIS 81SI 1SlS S2 S3 SurfacECG ECG mV Right jntricle Rght 'Ventricle V V l Downloaded from http://circ.ahajournals.org/ by guest on June 14, 2017 Atriumi Atrium: '1 m~V 1mV I~ -seco-nt Cec' D shock second shock D FIG 7. Electrogram recordings from shocks that resulted in fibrillation induction, one for each part of the study. In each part, the three channels shown from top to bottom are surface lead 11 ECG, right ventricular apex, and the electrogram recorded between the two shocking coils, one in the right atrium and the other in the great cardiac vein under the left atrial appendage. A, Example of an episode of ventricular fibrillation induced in part 1 of the study. There are two sinus beats to the left in the electrograms, followed by the initiation of the stimulus train at a cycle length of 250 milliseconds. After the eighth stimulus, there is a ventricular depolarization and the shock. The shock is delayed 20 milliseconds from the last depolarization. After the shock is ventricular fibrillation. B, Example of an episode of ventricular fibrillation induced in part 2 of the study. There are two sinus beats to the left in the electrograms, followed by the initiation of the stimulus train with eight paced beats at a cycle length of 300 milliseconds, followed 230 milliseconds later by the premature stimulus. After the premature stimulus, there is a ventricular depolarization and the shock. The shock is delayed 2 milliseconds from the last depolarization. After the shock is ventricular fibrillation. C, Example of an episode of ventricular fibrillation induced in part 3 of the study. There are two sinus beats to the left in the electrograms, followed by the initiation of the stimulus train with eight paced beats at a cycle length of 300 milliseconds, followed 550 milliseconds later by the first premature stimulus and 260 milliseconds later by the second premature stimulus. After the second premature stimulus, there is a ventricular depolarization and the shock. The shock is delayed 2 milliseconds from the last depolarization. After the shock is ventricular fibrillation. D, Example of an episode of ventricular fibrillation induced in part 4 of the study. The animal is in atrial fibrillation, and after a cycle length of 240 milliseconds, a shock is delivered, delayed 100 milliseconds from the V wave. After the shock is ventricular fibrillation. a preceding ventricular cycle length .300 milliseconds are associated with a significantly increased likelihood of ventricular proarrhythmia. All episodes of ventricular fibrillation seen during this study occurred when the preceding cycle length was c300 milliseconds, whereas no episodes of ventricular fibrillation were seen when the preceding cycle length was >300 milliseconds. We showed that with appropriate synchronization, which has previously been thought adequate to avoid the vulnerable period, ventricular fibrillation could occur if the preceding cycle length was c300 milliseconds. There was no correlation between shock strength and the occurrence of ventricular fibrillation. Although there were 4 episodes of ventricular fibrillation out of 24 testing protocols for group A and 7 out of 22 for group B, this difference was not significantly different overall (P>.15), nor was it significantly different when groups within any part, 1, 2, 3, or 4, of the protocol were compared (P>.15). Atrial Defibrillation Thresholds The atrial defibrillation thresholds seen in this study are comparable to the thresholds seen by others for similar left to right atrial vectors in sheep.10-'2 Like the previous studies, this study shows that in the sheep model of atrial fibrillation, atrial defibrillation can be achieved with transvenous electrodes with mean shock energies of < 1 J. The method of determining atrial defibrillation threshold was different in this study than for others.10'11 In this study, we determined the lowest-intensity shock required to defibrillate the atria and then delivered 20 shocks to estimate the percent successful defibrillation at this intensity. Techniques used by other investigators tend to estimate the 50% successful defibrillation point on the dose-response curve. At the lowest intensity that would defibrillate the atria, we found that the mean percent success was 52% for group A and 48% for group B (NS, P<.05). Although there was a rather large standard deviation in the measurement of these values, this method, on average, estimates the 50% point on the dose-response curve. Additionally, the mean percent success of 50% with a large standard deviation using a technique designed to find the lowest-intensity shock necessary to defibrillate the atria might suggest that the dose-response curve for atrial defibrillation is quite 420 Circulation Vol 89, No 1 January 1994 steep in comparison to the 10-V steps used in this protocol. Downloaded from http://circ.ahajournals.org/ by guest on June 14, 2017 Mechanisms of Ventricular Fibrillation Induction Three possible mechanisms exist to account for induction of ventricular fibrillation by internally delivered cardioversion shocks. One possible mechanism is lack of synchronization of the cardioversion shock with respect to intrinsic ventricular depolarization, resulting in the shock being delivered during a T wave. All shocks in this study were appropriately synchronized to ventricular depolarizations; therefore, this mechanism does not apply to the episodes of ventricular fibrillation induced in this study. A second potential mechanism is if a shock is synchronized to a ventricular depolarization that occurs before complete ventricular repolarization from the previous beat. From a surface electrogram, this may appear as an R on T. In our study, 9 of the 11 episodes of ventricular fibrillation appear to have been caused by this mechanism. All of the episodes were in parts 1, 2, and 3. Sample electrograms from these episodes are shown in Fig 7A, 7B, and 7C with sensed V wave-toshock delay of either 2 or 20 milliseconds. For all these episodes, it was evident from the right ventricular electrogram that the ventricular depolarization that triggered the shock was encroaching on the terminal portion of the preceding T wave. Others have observed a higher incidence of shockinduced ventricular fibrillation with shorter cycle lengths in both dogs and humans during transvenous cardioversion of ventricular tachycardia.1415 The investigators concluded that for tachycardia cycle lengths <200 milliseconds, transvenous cardioversion shocks would frequently accelerate the tachycardia or induce ventricular fibrillation. Although the results in those studies were similar to the present study, the mechanisms may be somewhat different because the ventricular tachycardia in the previous studies probably involved intraventricular reentry. Nathan et a18 described the induction of ventricular fibrillation from a 5-J shock delivered to right heart catheters near the onset of the QRS in a patient undergoing atrial defibrillation. The patient had an apparent rapid ventricular response based on the figure showing the intracardiac electrograms. They suggested that the mechanism of induction may be similar to that described by Jackman and Zipes14 for ventricular tachycardia. These reports are under conditions similar to part 1 of our study, in which the ventricular response in tachycardia is rapid and regular. Both the cycle length and dispersion of ventricular refractoriness have been associated with the vulnerability of the ventricles to reentry. These effects could increase the likelihood of ventricular proarrhythmia for synchronized shocks delivered to the terminal recovery phase of the preceding beat. In humans with long QT syndrome, long/short cycle lengths frequently precede episodes of ventricular arrhythmias.16 In patients with suspected sudden death, long/short and short/long cycle lengths frequently induce tachyarrhythmias and may predict the method of induction of spontaneous episodes.17-20 El-Sherif et a12' showed, in canine infarcted hearts, that reentry was more predominant when a short/long/short stimulation sequence was used. In atrial fibrillation, the irregular ventricular response and the predominance of short cycle lengths are an ideal setting for intraventricular reentry. In parts 1, 2, and 3 of this study, we systematically generated a spectrum of cycle lengths that facilitated shock induction of ventricular fibrillation. This study showed that short cycle lengths increased the likelihood of ventricular fibrillation induction in normal sheep hearts. The third mechanism pertains to the delay of shock delivery from the sensed ventricular depolarization and the effect of irregular and rapid cycle lengths on the refractory period of the ventricle and therefore the time from depolarization to the vulnerable period. Dunbar et a19 observed that transvenous defibrillation shocks delivered 116 to 180 milliseconds after the onset of the QRS were associated with nine episodes of induced ventricular fibrillation seen in their study. In part 4 of our study, two episodes of ventricular fibrillation were induced after short intervals with 100-millisecond sensed ventricular depolarizations-to-shock delivery delay. In these two episodes (Fig 7d), the shocks were delivered during the early upstroke of the T wave, in the right ventricular electrogram, with some of the myocardium apparently in a state of vulnerability. This relatively short recovery time of some of the myocardium may be attributed to the dispersion of refractoriness associated with the short/long/short preceding cycle lengths.22 Relative Risk of Ventricular Fibrillation Induction Previous studies done on low-energy transvenous cardioversion of atrial fibrillation have reported only minimal occurrence of ventricular fibrillation,8-10 whereas recent studies of higher-energy cardioversion of atrial fibrillation with shocks delivered between an intracardiac electrode and the chest wall have reported no episodes of ventricular fibrillation.23 In our study, in which producing ventricular proarrhythmia was the objective, only 11 episodes of ventricular fibrillation were induced. Eight of the 16 sheep in this study had no episodes of ventricular fibrillation despite having half of the test shocks delivered on intervals <300 milliseconds. It is apparent that even with conditions ripe for the induction of ventricular fibrillation (short and irregular cycle lengths with a dispersion of ventricular refractoriness), the induction of ventricular fibrillation is relatively rare. This study clearly showed that the risk of ventricular fibrillation induction was significantly higher when the preceding ventricular cycle length was 5300 milliseconds. In fact, no instances of ventricular fibrillation occurred when the preceding cycle length was >300 milliseconds. It is logical to assume that if enough time has elapsed since the last beat for all ventricular cells to fully repolarize, none of the ventricular myocardium should be vulnerable. This hypothesis is supported by the work of Elharrar and Surawicz,24 who showed, in canine myocardium, that ventricular action potential durations approached a maximum of 330 milliseconds at long preceding cycle lengths. Previous studies on the occurrence of ventricular fibrillation after atrial cardioversion have been performed with transthoracic cardioversion and found that shocks that were poorly synchronized with the R wave would consistently result in ventricular fibrillation when the shock fell during the T wave, the ventricular vulner- able period.1-5 With the advent of synchronized defibril~ Ayers et al Rate Effect on Atrial Defibrillation Proarrhythmia Downloaded from http://circ.ahajournals.org/ by guest on June 14, 2017 lators, the fear of ventricular fibrillation was decreased but not abolished for cardioversion of atrial fibrillation. Rarely is the association made between transthoracic cardioversion of atrial fibrillation and the occurrence of ventricular fibrillation. There may be several reasons for this low occurrence rate. First, as indicated previously, the induction of ventricular fibrillation by atrial defibrillation shocks is indeed rare, making a systematic study of its occurrence difficult. Second, most patients undergoing cardioversion are receiving medications to control their ventricular response to the atrial fibrillation, making shorter cycle lengths less frequent. Last, the energy typically used for transthoracic cardioversion is at or near the energy required to defibrillate the entire heart and is therefore unlikely to induce ventricular fibrillation. Implications This research has implications for the feasibility of transvenous atrial defibrillation with either an external or implanted atrial defibrillator. This study confirms the work of others1",12 that low-energy transvenous atrial defibrillation is feasible when both atria are interposed between the electrodes. The concept of an implantable atrial defibrillator has been raised by Levy and Camm,25 who discussed the critical paths in development of this device. One point discussed pertained to defibrillating the atria without ventricular arrhythmia induction. This study shows conditions under which transvenous cardioversion of atrial fibrillation can result in ventricular fibrillation. Shocks synchronized to sensed ventricular depolarizations preceded by a cycle length <300 milliseconds can cause ventricular fibrillation and therefore should be avoided by a transvenous atrial defibrillator. Fortunately, the distributions of cycle lengths during atrial fibrillation provide frequent cycle lengths longer than those shown in this study to be arrhythmogenic even when the mean heart rate is rapid.26 Although untested, a similar technique of precluding shocks when preceding cycle lengths are short may be applicable for transthoracic cardioversion as well. Limitations As with all animal studies, there are limitations to the animal model. First, in this study, the sheep was used to model human atrial fibrillation and ventricular vulnerability. It must be noted that although sheep and human hearts are similar in size and atrial fibrillation characteristics,26 undetected differences may exist. In addition, these were normal hearts not under the influence of any disease states that cause atrial fibrillation in humans, such as ischemia, atrial dilation, hypertension, etc. Also absent was the effect of long-term atrial fibrillation on the ventricular response, such as tachycardia-induced cardiomyopathy or a decreased ability of the ventricles to compensate for the rapid and irregular rates present in some of the human atrial fibrillation population. Additionally, the interactions of many of the drugs used to treat atrial fibrillation may affect the vulnerability of the ventricles or alter the atrial and ventricular defibrillation and fibrillation thresholds. Testing the influences of these other factors was beyond the scope of this study and requires additional evaluation. Second, ventricular pacing was used to mimic supraventricular influences of atrial fibrillation during 421 three parts of this study. Although many of the aspects of atrial fibrillation were simulated with ventricular pacing, the activation sequence was different with pacing. The effects of ventricular pacing, which could be considered analogous to premature ventricular complexes, should, in theory, increase the likelihood of ventricular fibrillation induction, since ventricular pacing delays conduction, resulting in prolonged depolarization and an increased dispersion of refractoriness.27 Additional testing with supraventricular pacing or a combination of supraventricular sensing and ventricular pacing, S, sensed with S2 and S3 ventricular paced, merits further study. Last, there was only limited randomization of the parts of this protocol. Although parts 1 through 3 were performed in random order, they always preceded part 4. Intervals within any part were not randomized. This lack of randomization was caused by our concern that many episodes of ventricular fibrillation would increase the risk of death of the animal, preventing completion of the protocol, or that multiple episodes of ventricular fibrillation would compromise the normal physiology of the model. We therefore conducted the study according to standard electrophysiological methodology in which prematurity was gradually increased. For this reason, the protocol for any of parts 1, 2, 3, or 4 was stopped after induction of ventricular fibrillation. For episodes of ventricular fibrillation that were probably caused by an R-on-T situation and a shock delay of 20 milliseconds, it can only be inferred that a shock delay of 2 milliseconds would have also caused stimulation in the vulnerable period and induced ventricular fibrillation, although this was not actually observed. In part 4, the short preceding cycle length and a shock delay of 100 milliseconds probably induced ventricular fibrillation by vulnerable-period stimulation of the T wave after the synchronized V wave. It is unknown in these two episodes whether a shorter delay would have induced ventricular fibrillation via an R-on-T shock mechanism, since no shorter delays were tested. This study shows that synchronized shocks with short preceding cycle lengths (<300 milliseconds) have a significantly greater risk of ventricular proarrhythmia. This study also confirms that low-energy transvenous atrial defibrillation of human-size sheep hearts can be accomplished with energies <1 J. Acknowledgment We thank Dr Susan Bernard for her technical assistance. References 1. Lown B, Amarasingham R, Neuman J. New method for terminating cardiac arrhythmias. JAMA. 1962;182:548-555. 2. Lown B. Electrical reversion of cardiac arrhythmias. Br Heart J. 1967;26:469-489. 3. Ferris LP, King BG, Spence PW, Williams HB. Effect of electric shock on the heart. Elec Eng. 1936;55:498-515. 4. Wiggers CJ, Wegria R. Ventricular fibrillation due to single localized induction and condenser shocks applied during the vulnerable phase of ventricular systole. Am J Physiol. 1940;128: 500-505. 5. Lown B, Kaidbey S, Perlroth M, Abe T. Comparative studies of ventricular vulnerability to fibrillation. J Clin Invest. 1963;42:953. Abstract. 6. Flaker GC, Blackshear JL, McBride R, Kronmal RA, Halperin JL, Hart RG, on behalf of the SPAF investigators. Antiarrhythmic drug therapy and cardiac mortality in atrial fibrillation. JAm Coil Cardiol. 1992;20:527-532. 422 Circulation Vol 89, No 1 January 1994 Downloaded from http://circ.ahajournals.org/ by guest on June 14, 2017 7. Kannell WB, Abbott RD, Savage DD, McNamara PM. Epidemiologic features of chronic atrial fibrillation: the Framingham study. N Engl JMed. 1982;306:1018-1022. 8. Nathan AW, Bexton RS, Spurrell RA, Camm AJ. Internal transvenous low energy cardioversion for the treatment of cardiac arrhythmias. Br Heart J. 1984;52:377-384. 9. Dunbar DN, Tobler G, Fetter J, Gornick CC, Benson DW, Benditt D. Intracavitary electrode catheter cardioversion of atrial tachyarrhythmias in the dog. JAm Coll Cardiol. 1986;2:1015-1027. 10. Powell AC, Garan H, McGovern BA, Fallon JT, Krishnan SC, Ruskin JN. Low energy conversion of atrial fibrillation in the sheep. JAm Coll Cardiol. 1992;20:707-711. 11. Cooper RAS, Alferness CA, Smith WM, Ideker RE. Internal cardioversion of atrial fibrillation in sheep. Circulation. 1993;87: 1673-1686. 12. Ayers GM, Ilina M, Wagner D, Kreyenhagen P, Bernard S, Alferness C. Cardiac vein electrode locations for transvenous atrial defibrillation. J Am Coll Cardiol. 1993;21:306A. Abstract. 13. Hoff HE, Geddes LA, McCrady JD. The maintenance of experimental atrial fibrillation by cholinergic factors. Cardiovasc Res Cent Bull. 1965;3:117-129. 14. Jackman WM, Zipes DP. Low-energy synchronous cardioversion of ventricular tachycardia using a catheter electrode in a canine model of subacute myocardial infarction. Circulation. 1982;66: 187-195. 15. Zipes DP, Jackman WM, Heger JJ, Chilson DA, Browne KF, Naccarelli GV, Rahilly GT, Prystowski EN. Clinical transvenous cardioversion of recurrent life-threatening ventricular tachyarrhythmias: low energy synchronized cardioversion of ventricular tachycardia and termination of ventricular fibrillation in patients using a catheter electrode. Am Heart J. 1982;103:789-794. 16. Kay GN, Plumb VJ, Arciniegas JG, Henthorn RW, Waldo AL. Torsade de pointes: the long-short initiating sequence and other clinical features: observations in 32 patients. J Am Coll Cardiol. 1983;2:806-817. 17. Denker S, Lehmann MH, Mahmud R, Gilbert C, Akhtar M. Divergence between refractoriness of His-Purkinje system and ventricular muscle with abrupt changes in cycle length. Circulation. 1983;68:1212-1221. 18. Denker S, Lehmann MH, Mahmud R, Gilbert C, Akhtar M. Facilitation of macroreentry within the His-Purkinje system with abrupt changes in cycle length. Circulation. 1984;69:26-32. 19. Denker S, Lehmann M, Mahmud R, Gilbert C, Akhtar M. Facilitation of ventricular tachycardia induction with abrupt changes in ventricular cycle lengths. Am J Cardiol. 1984;53:508-515. 20. Gomes JA, Alexopoulos D, Winters SL, Deshmukh P, Fuster V, Suh K. The role of silent ischemia, the arrhythmic substrate and the short-long sequence in the genesis of sudden cardiac death. JAm Coll Cardiol. 1989;14:1618-1625. 21. El-Sherif N, Gough WB, Restivo M. Reentrant ventricular arrhythmias in the late myocardial infarction period: mechanism by which a short-long-short cardiac sequence facilitates the induction of reentry. Circulation. 1991;83:268-278. 22. Janse MJ, van der Steen ABM, van Dam R, Durrer D. Refractory period of the dog's ventricular myocardium following sudden changes in frequency. Circ Res. 1969;24:251-262. 23. Levy S, Lauribe P, Dolla E, Kou W, Kadish A, Calkins H, Pagannelli F, Moyal C, Bremondy M, Schork A, Shyr Y, Das S, Shea M, Gupta N, Morady F. A randomized comparison of external and internal cardioversion of chronic atrial fibrillation. Circulation. 1992;86:1415-1420. 24. Elharrar V, Surawicz B. Cycle length effect on restitution of action potential duration in dog cardiac fibers. Am J PhysioL 1983; 244(Heart Circ Physiol 13):H782-H792. 25. Levy S, Camm J. An implantable atrial defibrillator. Circulation. 1993;87:1769-1771. 26. Ayers GM, Alferness CA, Illina MI, Wagner DO, Kreyenhagen P. Evaluation of a sheep model of atrial fibrillation. Am Heart J. 1992;124:839. Abstract. 27. Moore EN, Spear JF. Ventricular fibrillation thresholds: its physiological and pharmacological importance. Arch Intern Med. 1975; 135:446-453. Ventricular proarrhythmic effects of ventricular cycle length and shock strength in a sheep model of transvenous atrial defibrillation. G M Ayers, C A Alferness, M Ilina, D O Wagner, W A Sirokman, J M Adams and J C Griffin Downloaded from http://circ.ahajournals.org/ by guest on June 14, 2017 Circulation. 1994;89:413-422 doi: 10.1161/01.CIR.89.1.413 Circulation is published by the American Heart Association, 7272 Greenville Avenue, Dallas, TX 75231 Copyright © 1994 American Heart Association, Inc. All rights reserved. Print ISSN: 0009-7322. Online ISSN: 1524-4539 The online version of this article, along with updated information and services, is located on the World Wide Web at: http://circ.ahajournals.org/content/89/1/413 Permissions: Requests for permissions to reproduce figures, tables, or portions of articles originally published in Circulation can be obtained via RightsLink, a service of the Copyright Clearance Center, not the Editorial Office. Once the online version of the published article for which permission is being requested is located, click Request Permissions in the middle column of the Web page under Services. Further information about this process is available in the Permissions and Rights Question and Answer document. Reprints: Information about reprints can be found online at: http://www.lww.com/reprints Subscriptions: Information about subscribing to Circulation is online at: http://circ.ahajournals.org//subscriptions/