* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Late ventricular geometry and performance changes of
Electrocardiography wikipedia , lookup
Heart failure wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Jatene procedure wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Ventricular fibrillation wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
212
JACC Vol. 28, No. 1
Jsly 19%912-21
-
-
Late Ventricular Geometry and Performance Changes of Functional
Single Ventricle Throughout Staged Fontan Reconstruction Assessed
by Magnetic Resonance Imaging
MARK A. FOGEL, MD, FACC, PAUL M. WEINBERG,
MD, FACC, ALVIN
KENNETH
E. FELLOWS, MD, ERIC A. HOFFMAN,
PHD
Philadelphia,
J. CHIN, MD,
PmmyIvania
Obje&ws. We sought to test tbe bypotbesisthat late ventricular geomettyand performance changesoccur iu functional single
ventricles as they progress thmugb staged Footao reconstruction.
lkc@und. Indexesof ventricular geometry and perfonnanee
are important in evaluating the limctiooal state of the beart.
Magnetic resonanceimaging determines theseindexes in complex
vetttricular shapeswith mbtimal geometric assumptions.previous
s&dies have sbowu that 1 weeb after hemiFontan, the mass/
vohnae ratio markedly increases.
,$4&k& Mnltlpbase,q ultisllce,spin echo (n = 5) and cbte (n =
30) magnetic resonance bua&g was performed in 35 patientswitb a
ftmctiunaIsbtgle ventri& (1 weehto l2 years old) at various stages
of Fontau remnstructlon (15 bt the pre bemiFontanstage,11 after [6
to 9 months]the bemlFootmtprocedureand 9 alter [l to 2 years] the
Fontan proccdw). Volume and masswete c&dated at end-systole
ad emMbwtole. Ventricttl~ output wasthen obtained.Ventricular
c&mid motion wasalso calmdated.
ltewlts. No diieresce was noted (power >72%) from the pre
hemiFootan stage to 6 to 9 months after the bemiFontao procedure in (mean f SD) end-diastolic volume (lit4 + 24 vs. 123 +
40 cc/m’), mass (171 k 46 vs. 202 f 61 g/m’), ventricular output
(7.9 2 2.2 vs. 6.6 2 2.4 literslmia per q z) or ceotroid motion
(6.9 f 2.8 vs. 6.7 -C 2.6 mm/m’). Patients in the Fontan group
demonstrated a marked decreasein all indexes,indicating signifleant volume unloadbxg and decrease in mass and ventrlcnlar
performance. hlasslvolume ratio was not signiicantly different
among all three groups.
CorwtSa~~. No geometric and performance changesfrom tbe
volume-loaded stage are noted 6 to 9 months after the hemiFontan pmcedure; however,major changesoccur 1 to 2 yearsafter
the Footan procedure. The dramatic cbaoges in the mass/volume
ratio seenearly after the hemiFontan procedure were not detected
at 6 to 9 months. Furthermore, diminution of mass, volume and
ventricular performance are present at least 2 years after the
Fontan procedure.
(J Am toll Gudiol1996;28:212-21)
Ventricular ejection indexes and geometry (1) of functional
single ventricles involved in staged surgical reconstruction
leading to Fontan physiology (2), including hypoplastic left
heart syndrome (3,4) (stage I Norwood reconstruction, bidirectional superior vena cava to right pulmonary artery anaston&s (5) [“hemiFontan” procedure] and Fontan procedure)
has been assessedusing cardiac catheterization (6-9) echocardiography (10-13) and exercise testing (14,15). Measurement of ventricular volumes relied on geometric formulas and
assumptions (9-11,13,16-22) that are questionable in complex
geometric shapes of functional single ventricles.
Initially, the modified Fontan procedure was performed
directly (8), transforming a volume-loaded ventricle (pumping
to both systemic and pulmonary circulations) to an unloaded
one (23). The occurrence of hemodynamic deterioration postoperatively suggested an abnormality of diastolic compliance
(24,25), thought to be secondary to a sharp increase in
ventricular wall thickness to chamber dimension ratio (11).
The hemiFontan procedure (5,10,11) was then interposed 6
months after neonatal palliation, because the potential hemodynamic consequences of geometry changes induced by volume unloading might be better tolerated as a portion of the
systemic venous return fills the ventricle without passing
through the pulmonary circulation.
The purpose of this prospective study was to measure the
magnitude of the late changes in indexes of ventricular performance and geometry through all three stages of Fontan
reconstruction. We also evaluated the systolic motion of the
ventricular center of mass as an index of regional wail motion
and contractility. This study utilized magnetic resonance imaging because of its ability to acquire contiguous, parallel tomo-
From the Division of Cardiilogy, Department of Pediatrics and Department
of Radiology, The Children’s Hospital of Philadelphia and Departments of
Pediatrics and Radiology, The University of Penqlvania School of Medicine,
Philadelphia, Pennsylvania. Dr. Fogel has been ftmdcd ihrough a fellowship
gram of the Sootheastern Pennsylvania affiliate of :hti American Heart Asso&
rtion, Combohocken, Pennsylvania, and the American Academy of Pediatria
Section of Cardiology, Elk GmveVillage, Illinois. Dr. Hoffman has been funded
85 a~ established investigator of the American Heart A.w&ion, Dallas, Texas.
Manuwipt received May 26,1995: revised manuscript received February 26,
1996, accepted March 4.19%.
Ad&es for comesvondencc: Dr. Mark A. Fogel, The Children’s Hospital of
Phiiadclpbii Division of Cardiology, 34th Street and Civic Center Boulevard,
PhiladeIphia, Pcnngrlvania 19104.
0735-1o!l7/%/$15.00
PII so73s-10!47(%)001114?
JACC Vol. 28, No. 1
July Iwb:2:2-21
VtNTRlCUlAR
graphic images with a high temporal resolution and avoidance
of assumptions of ventricular shape. Its utilization for evaluating right and left ventricular geometry and performance has
been validated and used in multiple studies (26-35).
CHANGES WITH STACiED FONTAN
Tabte 1. Types
of
Functional Single
213
FOGEL ET AL.
KECONSTRUCTION
Ventricles
and Analysis
Hcmlfmran
Fontan
Group
Congenital Cardiac Le%n
Methods
Patients. We prospectively studied 35 patients with functional single ventricle at various stages of Fontan reconstruction who were followed up at The Children’s Hospital of
Philadelphia between April 1,199l and January 31,1993. Ages
ranged from 1 to 248 months (mean 2 SD 32.9 t 49.1 months,
median 14 months). Patients were stable enough to undergo a
l-h magnetic resonance imaging scan under sedation. Written
informed consent was obtained from all participants, their
parents, or both. The human investigations committee at The
Children’s Hospital of Philadelphia approved the study protocol on December 20, 1990. No patient had arrhythmias that
precluded imaging in the scanner.
Anatomic diagnoses were morphologic right ventricle in 29
and morphologic left ventricle in 6 (Table 1). Fifteen pre
hemiFontan patients, mean age 6.2 f. 2.3 months, had either
not been operated on or had undergone a systemic to pulmonary artery shunt @re hemiFontun group). Eleven had completed the hemiFontan procedure (5,10,1;) 6 to 9 months
previously (post hemiFonrun group) and 9 had completed the
Fontan procedure (2) 1 to 2 years earlier (Fontnn group)
(Table 2).
Angingrapby. Injections were made directly into the single
ventricle. Angiograms were obtained in the anteroposterior
and lateral projections and recorded at 60 frame& on 35-mm
film. Measurements were made utilizing catheter diameter as
an internal standard. The ventricular cavity was traced at
end-systole and end&stole in two orthogonal views, and the
area determined by planimetry and maximal apex-base axis
were measured by using a digitizing tablet and a PC utilizing
the software Digisonics (Digisoncs Inc.). The parallelepiped
(Lmax) method of Arcilla et al. (16) was used to calculate
ventricular volume, and the number of frames between the
end-diastole of one cardiac cycle and the enddiastole of the
next cardiac cycle was used to obtain the heart rate.
Magnetic resonance imaging. All patients were sedated
before imaging, and monitored with pulse oximetry, nasal
end-tidal carbon dioxide, electrocardiogram (ECG) and direct
visualization by television. All patients t&rated sedation
without incident.
Studies were performed on a 1.5tesla Siemens magnet. The
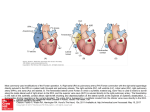
following scanning protocol was used (Fig. 1A):
1) A stack of coronal localizcrs was acquired to locate the
heart in the chest (Fig. lA, 1).
2) ECG-gated, T,-weighted transverse images spanning the
region of the heart were acquired (Fig. lA, II). The effective
repetition time (TR) = RR interval (range 350 to 800 ms),
echo time (TE) = 15 ms, number of excitations (NEX) = 3,
image matrix size = 256 X 256 pixels and slice thickness = 3 to
6 mm. These images were used to evaluate cardiovascular
by
Surgical Subgroups
HLHS
DOW
I. 1S.D D). MA. tuhPS. Dbl Ao
Arch, Lsvc -+ cs. SIP
bilai hemlFonran
2. 1S.D.L), Dextro, S-I vent.
CC-AVV, hypopla\t;c
RAW, iubPS. S.‘PFontan
3. KD,D). MA, ruhf’S.
unroofed CS unopcraled
4. {S.D.D}. ralvular and
subvalvalar PS. hypopla&
RV, unopcrated
I. IS.D.D), suhpulmonar) VSD.
muxtdar VSDs, atraddling
mitral valve. unopermed
Tricuspid atresin
I. ‘,S,D,D], TGA, UAA.
multiple muscular VSDs.
ASD, SIP hemiFontan
2. tS.D,Sl, S/P Fontan
2. (S,D,S), F’S,aneurym of
septum primum, SIP BTS
Pulmonary atresia;iVS
:. {S,D,S!. :&spid strnmic.
LAD aneurysm. LAD -+
RV fistula, SiP
hemiFoman
2 {S.D,S}, S,TFontan
TGA
1. 6.D.D). mitral hypoplaia.
severe PS. hypoplauic LV.
multiple muscular VSDS.
S/P Fontan
Isolated ventricular inversion
1. {S.I+S), SubPS.left
atriwentricxlar \akc
atresia. hypoplasfi- LV,
S,‘P BTS x ?
Single kh ventri&
1. {S.LX], hti uxta *iris
pulr~~naq at&a. SP
Fontan
10
3
8
1
I
I
-
I
-
-
I
-
-
-
Data presented arc number of patients. ASD = ostium sccundum atrial
~pml defect: bilat = bilateral; EtTS = mcdilied Blaiock-Tatig
shunt: CCAW
= criwzrw
atriownric&r
r&lions; Cs = cwmq
sinus: Dbl A0
Arch = double aortic arch; Dextm = dextrwardii: DDRV = dwbk+wrkt
right
ventricle; HLHS = hy$xstic
kh heart syxirome: IVS = intact ventricular
septum: LAD = left anterior descending corowq .uIq: UAA = left juxtapcsition of the auial appendages; UVC = kh superior vena cava: LV = kit
ventricle: MA = mitral atresia; PS = puhnonary stenasiz RAW
= right
atriorentricular valve: RV = right ventricubr, (S&S) = situ solitw of the
vi~~raandatria.ventrkularDbop,titasnwnaltyali@d~~arteties;
6.D.LJ)
= sanx as iSDS1 exap
D-m
w ma@%d great an&s
f~,Lt=samea(SDSIenceptLaaraposedumalpavd~tactrtierSm=
staNspaa;subP!i=slJbdvh~uenosa:slvent=vrperoinfti
wnaides;TGA=
tranq&mof&~tarteries:VSD=ve-sepcal
defect;-+=to.S%textfordelini&ofgnups.
FOGELETAL
214
VENTRlCIJLAR
CHANGES WITH STAGED FONTAN
Table 2. Study Patients With Sir&
JACC Vol. 2X. No. 1
July ly96:212-21
RECONSTRUCTION
Ventricle
Classified by Surgical Subgroup, Age, Weight, Height
and Body Surface Are+
Patients
(no.)
Surgical Subgroup
before hemiFoman
After hcmiFontan
Fontan
Total
I5
I1
9
35
Mean z SD
Median
5.7 + 2.0
38.2 5 42.4
74.6 5 69.4
32.9 2 49.1
6
22
37
I4
Weight
0%)
Height
(cm)
BSA
Cm’)
hlZ1.7
12.6 2 4.9
20.9 t 15.5
I I.8 f IO.0
66.3 f 8.0
89.1 f 19.4
104.91?-24.6
82.9 -t 23.2
0.33 2 0.07
0.55 2 0.17
0.76 ?r 0.35
0.50 + 0.26
Unless otherwise indicated, data presented are mean value f SD. BSA = body surface area.
and used
anatomy
as a localizer
for
subsequent
volumetric
(n =- 30) (Fig.
acquisition.
3) Volumetric acquisition. Off-axis coronal (Fig. IA, III)
multiphase,
multislice,
spin echo (n = 5) (Fig.
lA, TVA) or tine
ED
/
/
ES
i
CINE
ED
“
imaging
was performed
to obtain
a full
Figure 1. Magnetic resonance imaging calculation of ventricular geometry and performance
in a patient with a functional single ventricle
after a hemiFontan procedure. A 1, A stack of
coronal locabzers is acquired. II, Transverse
images that span the region of the ventricle are
obtained. Ill, OS-axis coronal images are obtained through the entire ventricle to yield a full
volumetric data set in either (lVA) multiphase,
multislice, spin echo (MPMS) or (Mk) tine
magnetic resonance imaging technicpA% B, By
using multiphase, multislice spin echo (left) or
tine magnetic resonance imaging (right) volumetric data sets, the epicardial and endocardial
borders of the ventricle are traced at enddiastole (ED) and end-systole (ES) on each
slice to yield indexes of ventricular
performance.
B
MULTIPHASE-MULTlSLlCE
IA, IVB)
volumetric
data set of the single ventricle at multiple phases of
the cardiac cycle. End-diastole was the first image after the R
wave, and end-systole
was the image in which the semilunar
ES
JACC Vol. 28, No. I
July 19!J6:212-21
VENl’RlCULAR
valve closed. In spin echo sequences,
TR. TE and distance
between slices were determined
as before, NEX = 2, matrix
size = 256 x 256 pixels. Contiguous
slices were obtained with
acquisitions
concatenated
to obtain each slice at each phase
during systole and early diastole (8 to 10 phases). In tine
sequences, TR = 50 ms, TE = 12 ms and slice thickness = 5
to 9 mm. The number of phases obtained was a function of the
RR interval
(usually
8 to 12), and the number
of slices
obtained varied from 10 to 12, depending on patient size. The
entire study was obtained in I hr. Respiratory
gating was not
performed,
although a respiratory
compensation
package was
used. A small error in our measure of ventricular
volumes may
be due to slight image blurring caused by lack of respiratory
gating.
Data analysis. Images were downloaded from the Magnetom onto a Sun SPARC station 10 (Sun Microsystems).
VIDA
(Volumetric
Image and Display Analysis)
(36), a software
package developed in our laboratory,
was used to manipulate
the images.
q
Standard variables of ventricular geometry aud perforauce. The ventricular epicardial and endocardial borders on
each slice at end-diastole
and end-systole
were traced with
cursor and mouse, excluding
semilunar
and atrioventricular
(AV) valves. Computer-aided
refinement
was performed,
and
the area was obtained
by counting
the number
of pixels
enclosed
by the borders
(Fig. IB). The areas were then
converted
to mm2 from pivets and then summed over the
entire ventricular
cavity by using the off-axis coronal slices. The
volume was obtained by
Ventricular
volume (cc) =
c
i=l
Area, X Slice, thickness,
[l]
CHANGES WITH STAGED FONTAN
FOGEL ET AL.
RECONSTRCiCTlON
215
Ventrkuiar center of mass motion. The centrgid of the
area of the ventricle
on each slice at end-diastole
and endsystolic was obtained by counting the pixels within the border.
The centroid of the volume at a given phase was determined by
weighting the centroid of the volume of the ventricle on each
slice at a given phase and dividing by the entire volume:
Venentriculhr center of marr
where X = horizontal axis on the slice, Y = vertical axis on the
slice and Z = azimuthal axis.
The distance a centroid moves between phases n and n + 1
(i.e., end-diastole
and end-systole)
was
Distance centroid
moved
= ,(x,-r
-~)‘i(Y”_,-Y,)‘~(zj-,-~)‘.
[7]
This distance was projected
anto the anteroposterior,
eral and superoinferior
planes using a two-dimensional
form (Fig. 2) (37):
sin 8
COS@
COS@
-sin8
lattrans-
ok-, -X2’
/I C&-,-&J
where areai = area of the ventricular
cavity LI slicei, and the
sum is over the entire ventricular
cavity. Ventricular
mass was
calculated
by the following formula:
Ventricular
mass (g) = 1.04 X (VoIume,prard,a,h.r,
Y n i , - Y. = Lpcroinferior
- Volumermkurd,rl
hrd I4
where 1.04 = specific
calculated as follows:
gravity
of muscle.
Ejection
indexes were
Stroke Volume (cc) = Cavity volume,,,,4w,olc
- Cavity volume,,..,,ti.
Stroke volume
Cardiac index (liters/min per mZ) = --~odysu~~;-
x
[3]
Heart rate
~.
PI
End-diastolic volume - End systolic voiume
Ejection fraction (o/c) = ~-~~~iast~?li------~---~-x loo.
IS]
All variables were indexed to body surface area (except for
ejection fraction. Cardiac index is already indexed) to make all
age and gender groups comparable
(8-11,13,15JO,21).
distance,
where 8 = angle the off-axis coronal plane (the plane in which
Z:lta were acquired)
makes with the lateral plane. Distances
*gere indexed to body surface area. For displacement
of the
-.;nter of mass, the absolute values of the distances were used.
Statistics.
Comparisons
between two means were made by
using the unpaired, two-way, Student’s I test and the Wikoxon
ranked sum test. Comparisons
among the three surgical sub
groups were made with one-way
analysii of variance
and
pairwise comparisons
made by using the Scheffk F test or
Fisher protected
least squares (38). All measurements
are
given as mean value ? SD. fntraobserver
variability
was
determined
by replicate
measures
using the coefficient
of
variability.
A single trained observer
performed
all image
analysis steps. The significance level was p < 0.05. This analysis
was performed
on a Macintosh
llci using Statview II v. 1.03
(Abacus Concepts)
and power calculations
were performed
with JMP v. 3.1 (SAS Institute
Inc.). The Bland and Altman
FOGEI. ET Al..
VENTRIU II AR CHANGES WI1 H STAGiil> I-ONTAY
RI‘(‘ON5 I RI ~C-TION
LATERAL
Fire
2. Graphic reprcseniation of the twc+dimensional rramfurm:
The pat&t has a functional single vsntricie and has undqone
a
hemiFontan procedure. After obtaining the ccntmid motion in thrcedimensions. the distance was projected onto the .uucropostcritrr.
lateral and superoinfcrior
plants hy using rhc two-dimcnaional Ir,m\formation in Cartesian coordinaics jscc Methods;).
method (39) was used to compare
resonance imaging data.
angiographic
and magnetic
Results
Standard
variables
of ventricular
geometry
and performance. Simpson’s rule and the preceding
formulas
I and 2
(which were used for all calculations)
were used to calculate
mass and volume in the same data set in IO of the 3S patients
and no significant
diAerence
was noted. Figure 3, A to F,
displays the comparison
among all three stages of Fontan
reconstruction.
No significant
difference
was noted between
the pre and post hcmiFontan
groups for all measured variahlcs; however values were significantly
smaller (except for
ejection fraction and masslvolumc
ratio) in the Fontan group.
Values in the post hcmiFontan
group were higher than expectcd. Power the of the one-way analysis of variance was
end-diastolic
volume 72%. end-diastolic
mass X0%, stroke
volume 79%. ejection fraction 29%, ventricular
output Y9%
and mass/volume
ratio 12%.
The Fontan group had a lower md-diastolic
volume (-SOc+
smaller, Fig. 3A), end-diastolic
mass (40% smaller, Fig. 3B).
stroke volume (50% smaller, Fig: 3C), ejection fraction (26%
smaller. Fig. 3D) andventricular
output (68% smaller. Fig. BE)
than the other two groups. Although end-systolic
volumes were
not statistically
diffcrcnt
between the prc hemiFor,!an
and
Fontan groups (34.3 2 IO.9 and 28.5 2 5.2 cc/m’. respectively)
the post hemiFontan
group had a higher end-systolic
volume
(61.1 + 52.1 cc/m’, p = 0.05) than either of the other groups.
Noting that this study evaluates Irtfc findings. we found no
statistical difference in the mass/volume
ratio (Fig. 3F) among
groups. Kart rates were not statl’ltically different between the prc
and post hemiFontan g oups (I I6 + 21 vs. I I3 z? IY heats/min.
respectively)
hut were lower for the Fontan group (XS z IS
heats/min). pre\urahly
&cause these patients were older.
Ventricular
center of mass motion.
Figur4. A to C,
summarizes
differences
in the ccnlcr of mass motion between
groups. Total displacement
in three dimensions
(Fig. 4A,
power Y9Si) was signific;mtly
smaller in the Fontan group than
in the prc or post hcmihtntan
groups (2.6 min!mf vs. 6.9 and
6.7 mm/m’, rcspcctivcly.
a reduction ot -62% ). Whrn divided
into its component
parts and projected onto orthogonal
planes
(Fig. 2 and 4B). the reduction
in total displacement
was noted
to he due to antcropostcrior
(power 7O’i) and lateral (power
.(%) motion. whcrcas the supcroinferior
plane (power 75;)
snowed no difference.
Furthermore.
there was a strong coneIation (r = 0.Y I) between anteroposterior
and lateral displacement (Fig. K) in the Fontan group that did not exist in other
groups or between the superoinferior
and either the anteropost&
or the lateral plane.
Comparison
between magnetic
resonance
imaging
and
angiographically
derived variables.
Bccausc of the similar late
ventricular
geometry
and pcrformancc
in the prc and post
hrmiFontan
groups. WL‘ compared
the post hcmiFontan
data
with angiographic
findings. We performed
this analysis simply
ac a comparison
of magnetic
resonance
imaging with the
recognized
reference
standard
of angiography
in normalshaped ventricles
and the comparison
in no way implies a
helief that the sngiopdphically
derived variables
represent
true in vivo measures.
Seven of II patients
in the post
hemiFontan
group underwent
angiography
within I week of
magnetic resonance imaging and all variahles calculated
from
angiography
and magnetic resonance
imaging data sets wcrc
significantly
ditferenl
(p < 0.05). Angiographic
values were
consistently
higher than the magnetic resonance
imaging values for end-diastolic
volume (214.0 t 60.7 vs. 9Y.3 2 44.4
cc/m’), end-systolic
volume (58.5 5 41.0~~. 4X.9 5 40.2 cc/m’).
ejection fraction (74.0 t 11.2 vs. 55.8 t 15.9% ), stroke volume
(155.5 t- 41.9 vs. SO.3 t 12.5 cc/m’) and cardiac index (16.6 I?
S.3 vs. 5.4 +- I. I litersimin
pLr m’), respcctivcly.
Table 3 and Figure 5. A to C, use the Bland and A!tman
method (39) to demonstrate
the lack of agrc-,nent
between
magnetic resonance imaging and angiographic
measures. As the
negative numbers in the mean differences point out, angirjgraphic
measures crmsistcntly overestimated
the magnetic resonance imaging ~;alues (end-diastolic
volume hy I IS cc. ejection fraction by
18si and cardiac index by I !.I litcn/min
per m’). Ninety-live
percent confidence intervals of the differences show a wide range
of pobsihlc values. These dilfcrcnces
are important
enough to
cause prohlcms in Jinical interpretation.
Discussion
We have previously
dcmonstratcd,
nance imaging, that distinct regional
using magnetic
rcsomechanical
differences
JACC Vol. 28, No. 1
July 1996:212-21
VENTRlCULAR
FOGEL FF AL.
RECONSTRUCJJON
217
T
l-
-I- T
w”
Clut5drU2)
5
Figure 3. End-diastolic volume (A), enddiastolic mass(B), stroke
voluli;c (C), ejectionfraction (D), indexedventricular output (I’.) and
mass/volumeratro (F) are displayed for all three stages15 F.>,rtan
reconstruction.
Error bars represent standard deviation. No ~~+$4es
(exceptejection fraction) differed significantlybetween the prt dnd
posthemiFontaugroups,but all valuesof the pre andpost hemiFontau
groups were significantly larger (except for mass/volume ratio) than
those measwed in the Fontan group. There was no statistica; dtierence in the mass/volume ratio among surgical subgroups. BSA = !nxiy
surface area; CC/M’ = cubic centimeter per square meter; EDV =
end-diastolic volume; FSV = end-systolic volume: G/CC = grams per
cubic centimeter;HEM1 = hemiFontanprocedure:HR = beart rate;
UMIN/M* = liters per minute per squaremeterof bodysurfacearea;
SV = stroke volume. Surgical subgroup
the text.
CHANGES WITH STAGED FONTAN
designations
are descriid
in
exist at various stagesof Fortian reconstruction based on strain
measures, wall motion (40,41) and the integrated function of
the heart (37,42).
We hypothesized that ventricular geometry and performance should he different at various stages because of the
volume overload of the ventricle before the hemiFontan
procedure (23) compared with that after the hemiFontan
(5,lOJl) and Fontan (2) procedures. Further. Hotfman (42)
demonstrated that atrial fibriftation and, pe-haps, reduced
atria) compliance or loss of atria1 5ystole can cause an important ventricular afterload. Most Fontan reconstructions utilize
a noncompliant intraatrial polytetrafhtoroethyiene hafile that
may act similarly to cause increased afterload on the ventricle.
Final!y, each stage ~requires deep hypothermic circulatory
arrest with possible &rssof myocardial viability and remodeling.
Both left and right ventrictes are included, as Chin et al.
(11) noted. a similar change in magnitude in ventricular
geometry as these chambers progress through staged reconstruction.
FOGEL ET AL
VENTRICULAR
218
CHANGES WITH STAGED FONTAN
.
Fii
4. For all three surgical subgroups, total three-dimensional
ventricular center of mass displacement (A), the component parts to
this motion projected onto three orthogonal planes (B) and the
correlation behveen anteroposterior (AP) and lateral &AT) motion in
the Fontan group (C) are displayed. Error bars represent standard
deviation.
Patients
in the Fontan
group
demonstrated
significantly
smaller total displacement in three dimens.ms (A) than did patients iu
either the pre or the post hemiFontan group. When this motion was
broken
up into its component
orthogonal
parts and projected
planes (B) by the two-dimensional
onto the three
transformation
JACC Vol. 28, No. 1
July 1996212-21
RECONSTRUCTION
(Fig. 2).
we observed that this reduction in total displacement was due to
motion in the anteroposterior
and lateral planes, whereas the superoinferior plane showed no difference in motion among the three surgical
subgroups. These two planes displayed a high correlation in the Fontan
gmup (0. MfvlAf’ = millimeters per square meter of hody surface; other
abbreviations as in Figure 3.
Pre hemlPontan
versus Fontan groups. Our data are consistent with the physiology
of pre hemiFontan
and Fontan
groups
The volume-loaded
heart before the ‘hemiFontan
procedure
has significantly
greater
end-diastolic
volume,
stroke volume, ejection fraction and ventricular
output than
the volume-unloaded
heart after the Fontan procedure.
The
increased work causes ventricular
hypertrophy,
hence the increased mass in the pre hemiFontan
group. Although
the
end-diastolic
volume decreased more than mass between patients in the pre hemiFontan
group and patients 1 to 2 years
after the Fontan procedure,
the mass/volume
ratio was not
statistically
different. This finding is consistent with the observations of Chin et al. (I 1): a greater change in volume than in
mass in comparing
postoperative
hemiFontan
and Fontan
data. Further,
the similarity
of the mass/volume
ratio is
consistent
with the study by Gewillig
et al. (25) 3 years
postoperatively,
although they studied patients with a single
left ventricle who underwent
a right atrial to pulmonary
artery
anastomosis.
Pre versus post bemiFontan
groups.
Contrary
to what
would be predicted,
the volume-unloaded
post hemiFontan
data were not statistically different from the volume-loaded
pre
hemiFontan
data. Indeed, the post hemiFontan
values were
higher than would be expected
from a volume-unloaded
physiology.
Furthermore,
the finding of an increased
post
hemiFontan
mass/volume
ratio 5 days postoperatively
(10) and
immediately
afte: removing a chronic volume load experimentally (24) was not present in our study 6 to 9 months postoperatively. Gewillig et al. (24) also found that mass/volume
ratio
normalized
over a l-month period. That mass/volume
ratio did
not differ among surgical groups implies normalization
of this
immediate postoperative
increase as part of a compensatory
mechanism
of the single ventricle.
There are several possible reasons why the pre and post
hemiFontan
data were similar. Triedman
et al. (43) observed
aortopulmonary
collateral
flow in 6.5% of patients
with a
bidirectional
Glenn shunt (i.e., hemiFontan
procedure)
and
found that a history of a Blalock-Taussing
shunt (present in 9
of 11 patients
in our series) increased
the likelihood
of
collateral channels. In our study, five of seven patients in the
post hemiFontan
group with angiograms
displayed some degree of aortopulmonary
collateral flow. This flow may partially
contribute
to the volume overload which, in turn, may contribute to increased ventricular
performance
by way of the Frank
Starling mechanism
(1). Furthermore,
because heart rate in
the post and pre hemiFontan
groups was similar but significantly higher than that in the Fontan group, the Bowditch
effect (1) (increased
contractility
with increased
heart rate)
may play a role in the increased performance
of the ventricle.
Another possible explanation
for the high ventricular
output may be blood
flow distribution
in response
to the hemi-
Fontan physiology
(i.e., increased flow to the head and neck
vessels may cause a compensatory
increase
in total blood
volume or cardiac output to keep flow to the rest of the body
~constant). Therefore,
this may represent a physiologic
control
mechanism
whereby
the amount of pulmonary
blood flow
,demanded by the body modifies and controb
systemic blood
flow to some degree. In this instance, flow to the brain may
dictate the ventricular
output (determinants
of cerebral perfusion may be different from normal because ce~bralpe&.&n
is
dependent
on bath
the cerebral
as well as pulmonmy
vascular
JACC Vol. 28. No. I
July 199/x212-21
VEM’RICULAR
Table 3. Magnetic Resonance
inaging VXWS Angiographic
CHANGES WITH STAGED FOXTAN
Measures of Ventrccular
Geometry
Varidhle
EDV (cc)
EF (%)
CI (IitersImin per m’)
Difference’
(bias)
Differencest
-%I to31
-47 tn IO
-mtn
1.0
-115 t 73
-I8 I 14
--il.1 f 5.0
and Perfnrrnance
9% Confidcncc Intel%a!s
lean
219
FOG51. ET AL..
RtCO~STRLXllON
Ri&
Upper lrmlt of
*eement(r
Lower Limit of
Aercement!
-lh? to --47.3
-31 tn c
- IS.7 to 6.4
-13tn33
4.1 m 7.1
-?9.?ro 13.1
‘“rn
.-?’
‘Mean DitTerence = [Xy; I (MRI value, - Angiographyvalue,)~.
Wigerences = mean dstTerence z ?x SD. Biia = bias f ifO.fl?.Chl K SE where the SE k the
divided by the square root of n (n = 7). NJpper limit of agreement = upper bound of the 9% crmfiderw interval for the diUercnch - t(0.QS.h) *’ SE of the
95% contidence interval for the ditlerences (SE as defined above x \!3). !lLauer limit of a~eement = Iowa bound of the %G confidera interval f?- the differenca
minus t(0.025.6) x SE of the 95% confidence interval for the diUerences (SE as defined above X VJJ. Cl = cardiac i&s: EDV = enddiidic
vdrmt: EF = ejection
fraction.
SD
&nre 5. Method of Bland and Altman. Graphs display the difference
between magnetic resonance imaging (MRI)
and angiographic
(CATB) measures (magnetic resonancz imaging values minus angiographic values [MRI - CATH]) on the ordinate and the average of the
magnetic resonance imaging and angiographic measures on the abscissa for enddiastolic volume (A), ejection fraction (B) and cardiac
index (C). Mean values and mean values 2 2 SD are shown. Angiegraphic measures consistently overestimated magnetic resonance imaging values, as indicated by the negative numbers on the ordinate. I =
liters.
A
so
0
z
------a
----mwn+asIl
.
ifi -50
*
g -100
__-..__l--------*l--I
m
5 -150
e-200
l!
g-250 t
----------nwm-2sD
-.,““-loa
n&:ruRkcr~cc,
bs
7
,o------
mom
.
204
-------
man+wD
.
E
.
3 -‘O,
z
ii
g -Jo.
f
13
.
,-,-,-------fmm-a?n
-WC
30
90
Avomm
C
.
. - - ; - --.---*-----.----:----
IuR~Amyz
m-4
------,,-mean+astl
-0. ..
I
Or---
l
--.------.--------------
-,a
-
-
.
.
-10
.
-----------em
-24
7
-eoD
II
A~&~~~
resirnrnce).
The systemic and pulmonary
circulations
in patients after the hemiFontan
procedure
are not in series or in
parallel but a hyhrid of the two (i.e.. the pulmonary
circulation
is in series with the head and neck circulation
and that entire
system is in parallel to flow to the rest of the body). Finally. this
may represent
a timedependent
adaptation of the ventricle
and us control to chronic hypoxia. The explanation
is probably
a combination
of all these factors. with Bow redistribution
heing the most important
of these.
Angiogrophy
and magwtiic resonance imaging. Our angiographic data support our magnetic resonance imaging findings
(Table 3, Fig. 5, A to C) of increased volume. mass and cardiac
index in the post hemiFontan
group. Angiographic
data yield
even higher volumes and performance
than would be expected
from this group. As we stated earlier. our comparison
of
magnetic resonance imaging data with angiographic
data does
not imply a belief that the angiographicaliy
derived data
represent true in vivo physiology
but was performed
to compare magttet;c resonance
imaging witn a reference
standard.
Further,
it is trot unexpected
that the parallelepiped
(L,,&
method of Areilla and associates (16) would overestimate
volume and performance
information
because the geometric
basis of this method would ttot hold in the bizarre shapes of
functional
sir@ ventricles.
Ejeetiou fnctiea.
End-systolic
volume did not differ between the pre hemiFontan
and Fontan groups. and the difference in ejection fraction between these groups was due to a
difference
in end-diastolic
volume. This observation
may explain the continued
nontrivial
prevalence
of effusions
after
completion
of the Fontatt procedure
in patients who have had
a hemiFontan
procedure
(i.e.. completion
of the Fontan
procedure
still comprises
a volume change)). The tncrease in
end-systolic
volume after the hemiFontatt
procedure
despite
an essentialy
uttehanging enddisstolie
volume contributes
to
the small, ittsiiifieant
deerease in ejeetii
fraction and cardiac index. The ejeetiott fractions are probably normal for this
patient group because the high ma&volu~
ratio decreases
ventricular
eomplianee
and makes the ventricle
hard to fill,
although it is probabfy shortening
maximally.
Vetttriak
eeatrokd s&inn.
Ventricular
oentroid motion
is the result of both regional heterogeneity
of systolic svall
220
FOGELETAL.
VENTRICULAR
CHANGES WITH STAGED FfJNTAN
JACC Vol. 28, No. 1
July 1996:212-21
RECONSTRUCTION
motion combined with intensity of contraction. We (40,41)
have previously reported the heterogeneity of wall motion in
the single ventricle tbtoughout staged Fontan reconstruction.
This would predict a large centroid displacement. The markedly decreased three-dimensional centroid motion of the ventricle in the Fontan group compared with that in the other
groups underscores the presumed decreased intensity of contraction and concomitant finding of decreased ventricular
performance.
The intraatrial baHe may play a role in ventricular motion
by restricting the normal descent of the AV valve plane toward
the apex (42). It is made of polytetrafluoroethylene and sewn
into the posterior and lateral at&l walls to create a cylindrical
channel for inferior vena cava blood. The decrease in centroid
motion in the Fontan group in the anteroposterior
and lateral
planes and the high correlation between the two planes (Fig.
4C) may be a result of restricted ventricular motion by a
noncompliant material to the baffle attachment points. Because the apex is the most lateral portion of the ventricle,
restriction of motion iz this plane implies restriction to AV
valve descent in the Fontan heart. This is similar to data
reported from our laboratory (37) on center of mass motion
throughout the car/., , cycle of the entire heart volume, which
found a high corr ration (r = 0.91) between these two planes.
signiicance. “71~ importance of accurately determining
both ventricular geometry and performance cannot be overemphasized. The role of magnetic resonance imaging in this
regard has been validated and utilized in multiple studies
(26-35). Kirklin et al. (7) noted ventricular hypertrophy as a
risk factor for death after the Fontan procedure. Seliem et al.
(9) noted that patients with a “poor” outcome after the Fontan
procedure had a statistically higher mass/volume ratio than
those with a “successfulor good” outcome and Matsuda et al.
(44) noted an increased mass/volume ratio in those patients
with a low output state after the Fontan procedure. A high wall
thickness/cavity was observed postmortem in many of our
patients in the Fontan group (12), and Fontan himself and
coworkers (45) observed that chamber hypertrophy was a
significant risk factor.
Limitations.
Because magnetic resonance imaging measures fixed planes in space,through-plane motion of the heart
may allow a small amount of volume to go undetected at the
farthest extent in the plane perpendicular to the axis in which
the slices were obtained. Because we took an extra slice on
each side of the ventricle, we do not believe that this effect is
appreciable.
Volume was determined by summing the area of the
ventricle on each slice and multiplying by slice thickness. This
procedure assumesthat the thickness of the prescribed area
remains constant throughout the slice, which is not true. The
volume error, however, is presumed to be very small and may
be compensated for by sliceson the “concave” side of the heart
offsetting those on the “convex” side.
Our statistical tests could not make use of paired measurements. Clearly, such a paired measurement design is a necessary long-term goal of this study. Finally, because we have no
data regarding the compliance of the lungs of these patients
during tidal breathing during the scanning, we cannot fully sort
OUIrhis effect.
Conclusions.
No geometric and performance changes are
noted from the volume-loaded ventricle before the hemiFontan procedure to the ventricle 6 to 9 months after the
hemiFontan procedure; however, major changes occur 1 to 2
years after the Fontan operation. The dramatic changes in the
mass/volume ratio previously seen with other imaging modalities 5 days after the hemiFontan procedure were not detected
at 6 to 9 months.
Patients who have undergone the Fontan procedure have
diminution of both mass and volume at least 2 years postoperatively. These Fontan ventricles have a marked decrease in
the systolic center of massmotion in three dimensions, and the
significant decrease in the antercposterior and !ateral planes
reflects the decreased vigor of contraction BIIU,;lay implicate
the intraatrial baffle w a factor.
We arc gratefui to William I Nwwod, MD. PhD and Marshal Jacobs, MD, who
performed all the opcrationr. Robert Fogel. PhD. who provided statistical
assistance, and John Hofvd. R9. who provided programming expertise.
References
1. Yang SS. Bcntivogl~o 1.6, Maranhao V. Goldberg H. Assessment of
ventricular function. In. Yang SS, Bentivoglio LG, Maranhao V, Goldberg
H, editors. From Cardiac Catheterization Data to Hemodynamic Parameters. Philadelphia: FA Dwis. 19881189-255.
2. Fontan F, Baudet E. %rrjcal repair of tricuspid atrcsia. Thorax 1971%:
240-8.
3. Notwood WI, Kirklin JK. Sanders SP. Hypoplastic left heart syndrome:
experience with palliative surgery. Am J Cardiol 1980;45:87-90.
4. Norwood WI, Jag P, Hansen D. Physiologic repsir of aortic atresiahypoplastic left heart syndrome. N Engl J Med 1983:308:2.?-6.
5. Hopkins RA, Armstrong BE, Setwer GA. Peterson RJ. G:aam HN.
Physiological rational for a hidirectional cavopulmonary shunt. A versatile
complement to the Fontan principle. 1 Thorac Cardiovasc Surg 1985,90:
391-8.
6. Lang P, Norwood WI. Hcmcdynamic assessment after palliative surgety for
hypoplastic left heart syndrome. Circulation 1983;68:104-8.
7. Kirklim IK, Blackstone EH, Kirklin JW, Prifico AD, Bargeron LM. The
Fontan operation. Ventricular hypetlrophy, age and date of operation as risk
factors. J Thorac Cardiovasc Surg 1986;92:1049-64.
8. Farrell PE Chang AC. Murdisott KA, Bah JM. Nonvood WI, Murphy JD.
Outcome and assessment after the modified Fontan procedure for hypoplastic left heart syndrome. Circulation 1992;86:116-22.
9. Seliem M, Muster AJ, Paul MH, Benson W. Relation between preoperative
left ventricular muscle mass and outcome of the Fontan procedure in
ptients with tricuspid atresia. J Am Coil Cardiol 1989;14:750-5.
LO. Seiiem MA, BaEa JM, Vetter JM, Chen SL Chin Al, Nor-wood WI. Changes
in right ventricular geometry and heart rate early after hemi-Fontan
prcccdure. Ann Thorac Surg 1993;55:1558-12.
11. Chin AI, Franklin WH, Andrew BAA, Nomood WI: Changes in ventricular
geometry early after Fontan operation. Ann Thorac Surg 1993;56:1359-65.
12. Weinberg PM, Chin AI, Murphy JD, Pigott JD. Nowwd WI. Postmortem
echocardiigraphic and tomographic anatomy of hypoplastic left heart syn
drome after palliative surgery. Am J Cardiol 19es;5S:i228-32.
13. Graham TP, Franklin RCG, Wyse RKH, Gooch V, DeanBeld JE. f&i
ventricular wall strw. and contractile function in childhood: nxmal wdues
and camparison of Fontan repair versus palliitioo only in patients with
tricuspid at&a. Ciilation
1986;74 Suppl f&61-9.
14. tillers TM, Driscoll DJ, Mottram CD, Puga FJ, Schaf HV, Danielson GK.
JAW Vol. 28, No. I
July 1996212-21
VENTRICULAR
Exercise tolerance and cardiorespiratory respmse to exe&c before and
after the Fontan operation. Mayo Clin Proc l%‘)M:I48Y-Y7.
IS. Nir A. Drisall Dl, Mottram CD. et al. Cardiorespiratory respome to
exercise after the Fontan operation: a serial study. J Am Coli Cardiol
1993:22:216-20.
16. Arcilla RA. Tsai P, Thileniur 0, Rannigcr K. Angiographic method for
volume estimation of right and left ventricles. Chat 1971;ho:446-54.
17. Devereux RB. Reichek N. Fxhocardiographic determination of left wmricular mas in man: anatomic validation of the method. Circulation 197755:
613-g.
18. Devereux RB, A!onso DR. Lutas EM. et al. Echocardiogmphic asswment
of left ventricular hypertrophy: comparison to necropsy findings. Am J
Cardiol lY~57:45(1-8.
19. Onnasch DGW. Lange PE, Heintzen PH. Left ventricular muscle volume in
children and young adults. Pediatr Cardiol 19&1:5:101-6.
20. Graham TP. Jarmakani JM, Canent RV, Morrow MN. Left heart volume
estimation in infaw and childhood. Circulalion Iyll:43:x95-9oJ.
21. Nakano H. Left v&icolar
volume estimation in normal infants and
children. Ann Paediatoici Jaarmci 1977:Z39-48.
22. Troy BL Pombo 1. Rackley CE Measurement of left ventricular wall
thickness and mas hy echocardiography. Circulation lY7?:4S:M~?-1I.
23. Pasque MK. Fontan hemodynamirs. J Card Surg 1988;3:45-52.
24. Gewillig M. Daencn W. Aubert A. Van der Hauwaert L. Abolishment of
chronic volume overload. Implications for diastolic function of the systemic
ventricle immediately after Fontan repair. Circulation 19!QR6 Suppl ll:ll93-9.
25. Gewillig M. Lundstrom UR. Deanfield JE. et al. Impact of Fontan operation
on left ventricular size and contractility in tricuspid atresia. Circulntion
19YO81:118-27.
26. Bat LM, Katz J. Magnetic resonance imaging for quantification of right
ventricular volume in patients with puhwnaty hvpertension. J Thorac
Imaging 1993;8:92-7.
27. Culham IA, Vita DJ. Cardiac output by MR imaging: an experimental
ctudy comparing right ventricle and left ventricle with thermodilution. Can
hrsof Radio1 J 1!%8;3Y:247-9
28. Dell’ltalia LJ, Blackwell GG, Pearce DJ. Thorn B, Pohmt GM. Assessment
of ventricular volumes using tine magnetic resonance imaging in the intact
dog. A comparixm of meawement methods. Invest Radii1 l’#;?Y:l62-7.
29. Maddahi J. Crues J. Berman DS. et al. Noninvasive quantification of left
ventricular rryrrardial muscle mass by gated proton nuclear magnetic
resonance imaging. J Am Coil Cardiol 1987;1fl:6X?-92.
Xl. Katz J, Milliken MC, Stray-Gunderwn I, et al. E&x&ion
of human
myocardial mass with MR imaging. Radiology I9XX:lm 05-X.
31. Ostnega E, Maddahi J, Honma H. et al. Ouantificatiw cf left \mrricular
myocardial ma%\ in humans hy nuclear magnetic rcwxm~.~ imating. Am
Heart J 198y;ll7:444-i;?.
.
.
C~~ANGES win+
STAGEI)
FONTAN
FOGEL ET AL.
RECONSTRUC~ON
221
32. Semelka RC. Tome1 E. Wagner S. et al. Intentudv reorcxlucihilitv of
dimensional and functional m&wemena
between cinhma&ic
raon&e
studies in the morphologically ahmmml left ventricle. Am Heart J ImCl:l19:
1.%7-73.
33. Benjelkwn H, ianney GB. Kirk KA. Blackwell GG. Lotan CS, Pohos GM.
interstudy 1CprOdUCbility
of hiplane tine nuclear magnetic rerunance mea!urementc of lelr ventricular function. Am J Cardiil 1991:67:I413-20,
41. Bert LM. Katz 1. Kolb T. Cz.egle$ FP, Barn RJ. Direct quantification of
right and left ventricular volumes wth nuclear magnetic rewnanee imaging
in patients with primary pulmona~ hypertension. J Am Coil Cardii
lW?:19:I.HW-15.
35. Pattynama PM. Iamb HJ. Van der Velde EA. Van der Wall EE. de Roes A
Leh venlricular meacuremems uith tine and cpin-echo M9 imaging: a study
of reproducibility aith wriance component analyris. Radiilo@ 1993:187:
261-X.
.36. Hoffman EA. Gnanaprakasam II. Gupta KB. Hofurd ID, ::ug&ti&
SC.
Kulawiec RS. VIDA: an environmem for multidimenwnal timape di&
and analysis. Prcr SPIE 1992~lh6(,:694-71 I.
37. Fogel MA. Weinberg PM. Feikms KE. Hoffman
E.4 Mapetic rewnarre
imagmg of conctan, total heart wlume and utter of m&s in patient\ with
functional sin@ ventricle prirv to and aftcr staged Fontan prwedure. Am J
Cardiol 1993:7?:14%43.
38. Ott L. An Introduction to Sati\tical Mrthcdc and Data Anal!+. 3rd ed.
Boston: PWS-Kent. 19%:31.%8.
39. Bland JhL Altman DG. Statistical methods for awning agreement between
two methods of clinical measurements. Lancet 1’%1:307-IO.
40. Fogel MA. Gupta KB, Weinixrg PM. Hoffman EA Strain ind wll motion
anal!+ of single ventricle\ throughout staged Fontan reconrlrwtion using
magnetic resonara tagging [&tract].
J Am Coil Cardiol lYf3~21 Sup@
A:3A.
41. Fqel MA. Gupta KB. Weinberg PW. Hoffman EA. Regional wall m&on
and strain anrly+ across stages of Fontan recmwwtion
by magnetii
rewmnce tagging. Am J Physiol 199SZ69:Hll32-52.
42. Hoffman EA. Constancy of total heart volume: an imaging appnwch to
cardiac mechanics. in: Sideman S. Beyer R. editors. Imaging Meawrements
and Analysis of the Heart. New Yorkz Hemisphere Pohlishing I991:3-1%
43. Triedman .IK. Bridget ND. Maker JE. Lock JE. Prevalence and risk factors
for anrtopuhnonag collateral vessels after Fontan and hdirectmnal Glenn
prwcdurn.
J Am Coil Cardiol 15993:?2:?07-15.
JJ. Matuda H. tiwshima
Y. Kishimoto H. et II. Problems in the modified
Fonntan opemtion for ur~iventricular hsdrl of the right ventricular rlip.
Circulation IY87:76 Soppl 111:111-45-52.
45. Fontan F. Femandez G. Cow F. et al. The size of the polmona~ arteries
and the rewlt\ of the Fontan operation. J lhorac Cardiour Surg I%%iu:lm:
7’ I-21.