* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download follow up solids
Dislocation wikipedia , lookup
Density of states wikipedia , lookup
Low-energy electron diffraction wikipedia , lookup
Nanofluidic circuitry wikipedia , lookup
Heat transfer physics wikipedia , lookup
Strengthening mechanisms of materials wikipedia , lookup
Hydrogen bond wikipedia , lookup
Metastable inner-shell molecular state wikipedia , lookup
Halogen bond wikipedia , lookup
Pseudo Jahn–Teller effect wikipedia , lookup
X-ray crystallography wikipedia , lookup
Nanochemistry wikipedia , lookup
Semiconductor device wikipedia , lookup
Glass transition wikipedia , lookup
Crystallographic defects in diamond wikipedia , lookup
Condensed matter physics wikipedia , lookup
State of matter wikipedia , lookup
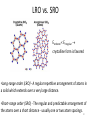
Colloidal crystal wikipedia , lookup
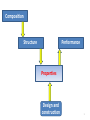
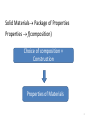
Composition Structure Performance Properties Design and construction 1 Solid Materials Package of Properties Properties f(composition) Choice of composition + Construction Properties of Materials 2 • Solid properties = f(Composition) • Solid properties = f(Atomic arrangement) Ex: diamond vs. graphite Composition alone can’t give the properties of a material, they are dependent on the atomic arrangement. pictures from wikipedia 3 Solid State Chemistry • Electronic structure of the elements holds the key to the understanding of the long range atomic order in solids; • Electronic structure of the atomic constituents and symmetry arguments are the criteria for the material selection process 4 1. What atoms are involved and their electronic configuration? 2. What types of chemical bonds are formed? 3. How are the atoms arranged in the crystal structure? 4. What is the symmetry of the crystal? 5. Do these arrangements promote certain mechanisms for electronic or atomic motions? 6. How do these mechanisms give rise to the observed properties? 5 Identifying solid materials by use of Atomic radii Chemical bond strengths Anisotropic atomic groupings Symmetry arguments Electronic band structure 6 Classification of solids based on bonding type 1. Ionic solids (e- transfer) 2. Covalent solids (e- sharing) 3. Metals (delocalized e-) 4. Molecular (Hydrogen bonds or Van der Waals forces) 7 1. Ionic Solids Electrostatic attractions between oppositely charged ions which is the same in all directions 1ei.e. NaCl Na + Cl [Na]+ + [Cl]- Ionic interactions are omni-directional and non-saturated The resulting low energy configuration: An ordered 3-dimensional network, a "crystalline" solid. 8 1. Ionic solids Have high degree of stability due to achievement of valence shell octets through bonding Non directional forces in such solids will result in highly ordered macroscopic bodies because of the repulsive forces in the cleavage plane ionic crystals are hard and brittle 9 Basic properties of ionic solids (1) Non–directionality of the electrostatic forces leads to an ordered solid (crystalline). (2) Octet stabilization makes the solid non–conducting (electrical insulator). (3) Stability will also most likely make the material transparent (high Eg) in the visible. (4) The magnitude of the attractive forces makes the solid melt at elevated temperature only. (NaCl-801ᵒC, MgO-2800ᵒC, CaF2-1418ᵒC) 10 2. Covalent Solids The electrons are shared between two interacting atoms in a molecular orbital (electron pairs); Electron-pair bonds are positioned to maximize the orbital overlap; Ex: sp3carbon in diamond sp2 carbon in graphite Bond directionality because the atomic and hybrid orbitals are quite definite and point in fixed directions 11 Properties of Covalent solids The directionality of the bond makes the properties to vary widely depending on the arrangement of the bonds diamond graphite sp3 hybridization of carbon Cubic structure The electrons are very tightly bound very strong bonds sp2 hybridization of carbon Hexagonal structure Strong covalent bonds in plane weak delocalized bonds normal to the planes the planes can slide over one another •Hard ( used as cutting tool) •Insulator (Eg=5.4eV) •Transparent to visible light •High refractive index •Lubricity ( used as lubricant) •Decent electronic conduction •Absorbs the photons and appears black 12 Diamond is the metastable form of carbon. The stable form is graphite! Diamond can be synthesized from graphite at high pressure Memorial diamonds from carbonized human remains by companies such as GemLife (US), Algordanza (CH), HeartIn diamond (Ru) 13 Diamond vs. Silicon Diamond – insulator Silicon – semiconductor (Band gap, Eg = 1.1eV) In Silicon, the Si atom is larger and the bond is longer (dSi-Si=2.35Å vs. dC-C=1.54Å; weaker bonds the e- are more easily liberated) smaller band gap and therefore semiconductor Graphite vs. Boron Nitride Graphite - conductor Boron Nitride – insulator In BN – two e- are only on N; the N atoms are separated by an B atom in the plane poor orbital overlap and therefore insulator dB-N=1.45Å dC-C=1.42Å In graphite – one unpaired e- is on each C atom good orbital overlap and therefore good conductor 14 3. Metals In metallic bond, the atoms loose their outer electrons to a common electron band that runs throughout the solid (e- delocalization). ion cores We may therefore visualize metals as a lattice of ion cores being held together by a gas of free electrons. gas of free electrons 15 Properties of Metals •The atoms pack together like spheres and the bonding is non-directional Planes slip is a common phenomenon in metals • Displacement of neighboring planes does not lead to charge effects and therefore malleability and ductility •High conductivity because the valence electrons never remain near any particular atom very long 16 4. Molecular solids 1. Van der Waals or secondary bonding results from the coulombic attraction between induced dipoles + - + - solid He(1-1.5K and 2.5MPa) Atomic or molecular dipoles Van der Waals solids have extremely low melting points 2. Hydrogen bonding – a special type of secondary bonding exists between some molecules that have hydrogen as one of the constituents ice 17 Directional vs. non-directional bonds In solids with directional bonds, the atoms cannot pack together in a dense manner, yielding to a low mass density [ (g/cm3)] density of covalently solids < than metallic or ionic ones; Density of solids - (g/cm3): •Ionic solids: NaCl (2.6 ) •Metals*: K (0.86); Ca (1.55); Sc (2.98); Ti (4.5) •Covalent solids: Diamond (3.53); graphite (2.23); SiC (3.21 ) * The fewer electrons lost to get full outer shells, the lower density 18 Classification of solids based on atomic arrangements: Classes Atomic arrangement Ordering type Ordered Regular Long range Disordered Random* Short range Name Crystalline “crystal” Amorphous “glass” *Not totally random 19 LRO vs. SRO Eordered < Eirregular crystalline form is favored •Long-range order (LRO) - A regular repetitive arrangement of atoms in a solid which extends over a very large distance. •Short-range order (SRO) - The regular and predictable arrangement of the atoms over a short distance - usually one or two atom spacings. 20 Transparent crystal vs. Opaque glass Diamond (Eg=5.4eV) Obsidian (volcanic glass: 70–75% SiO2, plus MgO, Fe3O4) Transparency: Eg>>3eV; Transparency is not a property linked to the long range atomic ordering of a solid; it is a function of the band gap of the solid; pictures form Wikipedia 21 Solids classification summary A. Based on bond type 1. Ionic solids 2. Covalent solids 3. Metals 4. Molecular solids B. Based on atomic arrangements I. Ordered solids = Crystalline II. Disordered solids = Amorphous 22 Crystalline solids • have characteristic geometric shapes Particles vibrate about their equilibrium positions NaCl diamond • have sharp melting points • are anisotropic by nature 23 Crystalline forms •Single crystal = the regularity of the pattern extends throughout a certain piece of solid Silicon crystal When cut or hammered gently it shows a clean fracture along a smooth surface. •Polycrystalline = the regularity or periodicity is interrupted at the grain boundaries* *grain boundary = where different aligned grains meet with each other 24 Single vs. Polycrystals • Single Crystals are anisotropic = properties vary with direction E (diagonal) = 273 GPa the degree of anisotropy increases with decreasing structural symmetry Fe E (edge) = 125 GPa • Polycrystals are isotropic = properties may/may not vary with direction 200 mm the measured property represents some average of the directional values Epoly Fe = 210 GPa From Callister 5e 25 Polycrystals Most engineering materials are polycrystals. Different color stands for different grain orientation • Each "grain” is a single crystal. • crystals are randomly oriented, overall component properties are not directional. • Crystal sizes varies (from 1 nm to 2 cm) 26 Crystals forms • Isomorphism = crystals of different composition with the same form Ex: halite (NaCl) fluorite (CaF2) sylvite (KCl) • Polymorphism = different crystal forms with the same chemical composition; allotropes Ex: r .t . Fe( BCC ) 1183K r .t . Fe( FCC ) high pressure C graphite( hexagonal) 1663K Fe( BCC ) Cdiamant( cubic) 27 Next • • • • • Introduction to Crystallography Bravais lattices Unit cell Crystal structure Characteristic of cubic systems 28