* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Survey
Document related concepts
Transcript
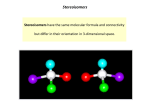
STEREOCHEMISTRY Dr. Sheppard CHEM 2411 Spring 2015 Klein (2nd ed.) sections 5.1-5.9, 8.4 Stereochemistry • Branch of chemistry concerned with the spatial arrangement of atoms in molecules • Stereoisomers: • Same molecular formula • Same connectivity • Different 3D orientation (cannot be converted via bond rotation) • Previously: • Cis and trans • Now: • Stereochemistry at tetrahedral centers • Enantiomers, diastereomers • E and Z Chirality • “handedness” • Has a mirror image that is nonsuperimposable • Example: hand • Example: sunglasses Chiral or not? Chiral or not? Chirality • A molecular example: • Try this with your model kit Achiral molecules • Are superimposable on their mirror images • Contain a plane of symmetry • Cuts through the middle of the molecule so that one half reflects the other half achiral chiral Enantiomers • Chiral molecules form enantiomers • Nonsuperimposable mirror images • Result from tetrahedral C (sp3) with 4 different substituents • This C is called a chirality center (or stereocenter, or asymmetric center) and are often marked with * • Examples of stereocenters: Enantiomers • Example: • (+) and (-)-lactic acid are a pair of enantiomers Identify the chirality centers Molecule Stereocenter? Plane of Symmetry? Chrial? Molecule Stereocenter? Plane of Symmetry? • Not all molecules with stereocenters are chiral! Chrial? Enantiomer Similarities and Differences • Same molecular formula, connectivity • Different 3D arrangement • Same physical properties (mp, bp, solubility) • Same spectroscopic properties (IR, NMR, etc.) • Same reactivity, in general • Products will have different stereochemistry • Only one will react with an enzyme (like a hand fitting in a glove) • Different designations (R vs. S) • Different optical activity Optical Activity • Rotation of plane- polarized light • Seen in chiral molecules • a = observed rotation; measured by the polarimeter • One enantiomer rotates light to the left a degrees • Levorotatory (-) • The other rotates light to the right a degrees • Dextrorotatory (+) Optical Rotation • Depends on polarimeter pathlength (l) and sample concentration (c) • Specific rotation [a]D is observed under standard conditions • l = 589.6 nm • l = 1 dm (10 cm) • c = 1 g/cm3 • (-)-Lactic acid has a [a]D of -3.82 • (+)-Lactic acid has a [a]D of +3.82 • What is [a]D of a 50:50 mixture of (-) and (+)-lactic acid? R and S designations • Used to describe 3D configuration about a chirality center • Not related to direction of optical rotation (+) and (-) • To designate R and S need to assign priorities to each group bonded to the stereocenter • Cahn-Ingold-Prelog system Priority Rules 1. Higher atomic number (of atom bonded to C*) = higher priority -Br > -Cl > -OH > -NH2 > -CH3 > -H 2. If 2 of the same atom are bonded to C*, look at atomic number of the next set of atoms • Continue process until first point of difference • Some more examples: Priority Rules 3. Atoms in double bonds count twice; atoms in triple bonds count three times Which substituent has the higher priority? a) -Br -Cl b) -CH2CH3 -CH(CH3)2 c) -CH=CH2 -CH2CH3 d) -CHO -CO2H e) -CH2OH -CH2CH2OH To designate R or S: 1. Locate chirality center 2. Assign priority to the 4 groups (1 = highest; 4 = lowest) 3. Orient molecule so substituent 4 is point away from you (with model or on paper) 4. Read the other groups 1→2→3 (draw arrow on paper) 5. Groups read clockwise = R; counterclockwise = S Example: 2-bromobutane Example: 2-bromobutane Rank the following groups in order of priority from highest (1) to lowest (4): -NHC(O)CH3 -OCH3 -OH -F Draw R and S stereoisomers for 2-hydroxypropanal: R and S stereoisomers for 3-methylhexane: • Hints: • Switch any two groups to draw the enantiomer • When substituent 4 is forward, 1→2→3 clockwise is S Classify these as chiral or achiral: How many chirality centers? Rotating a Tetrahedral Carbon • To rotate a carbon and not accidentally change the R/S designation, keep one substituent in the same place, and rotate the other three. • Make sure all three groups are rotating in the same direction • Do not switch two groups; this changes the R/S designation Classify these molecules as R or S: Determine whether the two structures in each pair represent constitutional isomers, enantiomers, or identical compounds. a) b) Fischer Projections • Another way of drawing tetrahedral carbons • Horizontal lines = out of page • Vertical lines = into page • Frequently used for chirality centers, especially if a molecule has more than one chiral center What is the relationship between these two molecules? Molecules With Multiple Stereocenters • Maximum # stereoisomers = 2n where n = # stereocenters # Stereocenters # Stereoisomers Stereoisomers 1 2 R S 4 (R,R) (S,S) (R,S) (S,R) 2 Example: 2,3-Pentanediol • Draw Fischer projections for the 4 stereoisomers • Carbon chain vertical, C1 at top Relationships • A and B are enantiomers • C and D are enantiomers • A and C, A and D, B and C, B and D are diastereomers Diastereomers • Stereoisomers that are not mirror images of each other • Different physical properties • With tetrahedral carbons, require at least 2 stereocenters • Cis-trans stereoisomers are also diastereomers Meso Compounds • Maximum # stereoisomers = 2n where n = # stereocenters • The # stereoisomers will be less than 2n when there is a meso compound • Meso compound • An achiral compound which contains chirality centers • Not optically active • The chirality centers typically are identical (have the same 4 substituents) and reflect each other in a plane of symmetry • Example: Another Example: 2,3-Butanediol • A = (2R,3R)-2,3-butanediol • B = (2S,3S)-2,3-butanediol • C = D = meso-2,3-butanediol • C and D are superimposable mirror images (the same molecule) • Relationship between enantiomers and meso? • Diastereomers Racemic Mixtures • aka Racemate, + pair, or d,l pair • 50% mixture of two enantiomers • Not optically active • Separation of enantiomers is difficult • Separation methods: • React with chiral compound to convert to a pair of diastereomeric salts, which can be separated by distillation, crystallization, etc. • Separate on chiral column • Separate with enzyme Applications of Stereochemistry 1. Stereochemistry of reactions • If a product has a stereocenter, is the stereochemistry all R, all S, or a mixture? • To understand details, need to look at mechanism (next) Applications of Stereochemistry 2. Reactions with enzymes • Receptors/enzymes react with only one enantiomer (like a handshake) • Limonene • R = orange odor • S = pine odor • Ibuprofen • R = inactive • S = active • D-Decalactone • R = porcupine emits to alert predators • S = coconut Thalidomide • How many chirality centers? • How many stereoisomers? • How was the drug administered? • What effect did this have on patients who used thalidomide? Francisco Goya Alkene Stereochemistry • Previously, cis-trans stereoisomers • Now, E,Z-designation of alkenes • Use E,Z instead of cis-trans when • More than two substituents on C=C • Heteroatoms on C=C • To assign E or Z: • Rank the two groups on each carbon of the C=C according to the Cahn-Ingold-Prelog priority rules • If the higher priority groups are on the same side of the C=C, the alkene has Z geometry • If the higher priority groups are on opposite sides of the C=C, the alkene has E geometry E and Z Configurations Classify these alkenes as E or Z: a) b) Name these alkenes: a) b) Isomerism Worksheet Next… • Organic reactions