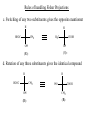* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Learning materials
Survey
Document related concepts
Transcript
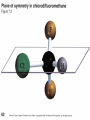
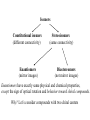
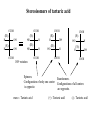
Stereochemistry Required background: Shapes of molecular structures Isomers, conformers Essential for: 1. Understanding of SN1, SN2, E1, E2, and other reaction mechanisms 2. Stereochemistry of carbohydrates, proteins, and nucleic acids Outline 1. Chirality, optical activity 2. Fisher projections. R-,S-nomenclature 3. Stereoisomers 1. Chirality, optical activity 2. Fisher projections. R-,S-nomenclature 3. Stereoisomers Chirality – is a property of an object to be different from its mirror image. In other words, the object and its mirror image are noncongruent. Example: A human hand is chiral, since the right hand and the left hand are different. Enantiomers – are noncongruent mirror images of molecules Chiral objects can not contain certain elements of symmetry, for instance, plane of symmetry (most common), center of symmetry or some other elements (rare cases beyond our course). Optical activity – is the ability of chiral compounds to rotate the plane of polarization of light. 1. Chirality, optical activity 2. Fisher projections. R-,S-nomenclature 3. Stereoisomers Which structural elements bring chirality to a molecule? a. Chiral center (sp3 – carbon with four different substituents) b. Other elements (beyond our course) The language for stereochemistry of molecules with chiral centers – Fisher Projections Rules of handling Fisher Projections a. Rotation by 180o in the plane of paper gives the identical compound H OH HOOC CH3 H3C COOH OH H (R)- (R)- b. Rotation by 90o in the plane of paper gives the opposite enantiomer H HOOC COOH CH3 HO H OH CH3 (R)- (S)- Rules of handling Fisher Projections c. Switching of any two substituents gives the opposite enantiomer H HOOC H CH3 H3C COOH OH OH (R)- (S)- d. Rotation of any three substituents gives the identical compound H HOOC H CH3 HO COOH OH CH3 (R)- (R)- 1. Chirality, optical activity 2. Fisher projections. R-,S-nomenclature 3. Stereoisomers Isomers Constitutional isomers (different connectivity) Enantiomers (mirror images) Stereoisomers (same connectivity) Diastereomers (not mirror images) Enantiomers have exactly same physical and chemical properties, except the sign of optical rotation and behavior toward chiral compounds. Why? Let’s consider compounds with two chiral centers Stereoisomers of tartaric acid COOH COOH (R)H H (S)HO OH (S)- HO OH COOH (R)H (R)- H COOH o COOH H HO (S)OH (R)- H COOH 180 rotation Epimers. Configuration of only one center is opposite. meso - Tartaric acid COOH HO H H (S)- OH COOH Enantiomers. Configurations of all centers are opposite. (+)- Tartaric acid (-)- Tartaric acid