* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Lecture note Part II (Coordination Chemistry)
Bond valence method wikipedia , lookup
Oxidation state wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Cluster chemistry wikipedia , lookup
Hydroformylation wikipedia , lookup
Metal carbonyl wikipedia , lookup
Jahn–Teller effect wikipedia , lookup
Spin crossover wikipedia , lookup
Metalloprotein wikipedia , lookup
Electronic configuration of the 3d transition elements
Sc Ti V Cr Mn Fe Co Ni Cu Zn
4s
3d
2
1
2
2
2
3
1
5
2
5
2
6
2
7
2 1 2
8 10 10
Electronic configuration of the Lanthanides
La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Hf
6s
5d
4f
2
1
0
2
1
1
2 2
0 0
3 4
2
0
5
2
0
6
2 2
0 1
7 7
2 2 2 2 2 2 2 2
0 0 0 0 0 0 1 2
9 10 11 12 13 14 14 14
Definition:
A complex or coordination compound is a compound in
which an atom (called “central atom”) is bound to more
groups (called “ligands”) than expected with respect to
its charge and position in the periodic table.
The number of ligands around a central atom is called
the “coordination number”.
Rules for naming complexes
first in the names of a complex the ligands are named in alphabetic order
of the first character (there is no distinction between anionic and other
ligands)
followed by the name of the central atom
the number of ligands is indicated by greek numerals: mono, di, tri, tetra,
penta, hexa, hepta, octa, nona, deca
if necessary bis, tris, tetrakis, pentakis etc. may be used
for the central atom the following rules are used:
in a neutral or cationic complex the name of the metal is used followed by
an information on its oxidation state
in an anionic complex the name of the metal is used plus an suffix -ate
for some metals the latin name has to be used: -plumbate, -ferrate, argentate, -cuprate, aurate etc.
The names of the ligands are used with an suffix -o if the ligand is an
anion
-chloro, -hydroxo, -thio, -oxo, -nitrato, carbonato etc.
For neutral or cationic ligands the name of the ligand is used and
sometimes included in round brackets. In some cases special names
have to been used: aqua (H2O), ammine (NH3), carbonyl (CO),
nitrosyl(NO)
Examples: Potassiumtetrafluorooxochromate
Tris(ethylendiamin)cobalt(III)sulfate
Tetrakis(trifluorphosphin)nickel(0)
Tetraammincopper(II)chloride
Rules for writing formula of complexes
•complexes are enclosed in square brackets
•first the name of the central atom is given
•followed by first the anionic ligand and then the neutral ligands;
within each group they are alphabetically ordered according to
the first character of their formula
Examples:
[PtCl2(C2H4)(NH3)]
K2[PdCl4]
[Co(en)3]Cl3
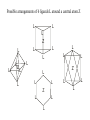
Possible arrangements of 6 ligands L around a central atom Z
L
L
L
Z
L
L
L
L
Z
L
L
L
L
L
L
L
L
Z
L
L
L
L
Z
L
L
L
L
L
Possible arrangements of the ligands in an octahedral
complex of composition [ZL4X2]
X
L
L
Z
L
X
X
L
L
L
Z
X
L
L
Possible arrangements of the ligands in a trigonal prismatic
complex of composition [ZL4X2]
X
L
X
L
L
L
Z
Z
L
L
X
L
X
L
L
L
L
Z
X
L
X
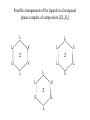
Possible arrangements of the ligands in a hexagonal
planar complex of composition [ZL4X2]
L
L
L
L
X
Z
L
X
Z
X
L
L
L
L
X
X
Z
X
L
L
L
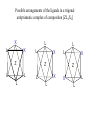
Possible arrangements of the ligands in a trigonal
antiprismatic complex of composition [ZL4X2]
X
L
L
X
L
Z
L
X
L
Z
L
L
L
Z
X
L
L
X
L
X
L
Examples:
[Co(NO2)6]3-
[PtCl6]2-
[Ag(NH3)4]+
Co3+
6NO2-
Pt4+ 74 e6Cl- 12 e86 e-
Ag+ 46 e4NH3 8 e54 e-
24 e12 e36 e-
but
[Cr(NH3)6]3+
[Ni(NH3)6]2+
[CoCl4]2-
Cr3+
6NH3
Ni2+ 26 e6NH3 12 e38 e-
Co2+
4Cl-
21 e12 e33 e-
25 e8 e33 e-
Many elements form complexes which do not obey the
EAN rule.
The EAN rule is helpful for organometallic compounds and
carbonyl complexes, which obey in most cases this rule:
[Cr(CO)6]
Cr
6CO
24 e12 e36 e-
[Fe(CO)5]
[Ni(CO)4]
Fe
26 e5CO 10 e36 e-
Ni
4CO
28 e8 e36 e-
metals with odd numbers of electrons form dimers or are reduced
or oxidized
[Mn(CO)6]+
oxidation
[Mn(CO)5][Co(CO)4]-
reduction
[Mn(CO)5]
dimerization
[Mn2(CO)10]
[Co(CO)4]
dimerization
[Co2(CO)8]
unknown
reduction
unknown
oxidation
[Co(CO)5]+
Similarly the formation of olefin complexes and
metallocenes may be explained by the EAN rule:
olefines donate 2 electrons /double bond
ethylene
2
butadiene
4
benzene
6
cyclopentadienyl radical 5
[Fe(C5H5)2]
Fe
26
2 C5H5· 10
36
[Mn(CO)5C2H4]+
Mn+
24
5 CO
10
C2H4
2
36
[Cr(C6H6)2]
Cr
24
2 C6H6 12
36
Bonding in co-ordination compounds
•
effective atomic number (EAN) rule
based on the octet theory of Lewis this is the first
attempt to account for the bonding in complexes
The formation of a complex was described as an acid base reaction according to Lewis
The sum of the electrons on the central atom (Lewis
acid) including those donated from the ligands (Lewis
base) should be equal to the number of elctrons on a
noble gas
Bonding in coordination compounds
•
valence bond theory
Linus Pauling made the first successful application
of bonding theory to coordination compounds
closely related to hybridization and geometry of
non complex compounds
the structures of complexes may be rationalized by
the following hybrid orbitals:
d2sp3 octahedral
dsp3 trigonal bipyramid
dsp2 square planar
sp3
tetrahedral
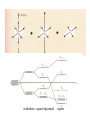
Crystal field theory
d- electrons in an octahedral field of ligands
octahedron
tetrahedron
distorted tetrahedron
tetrahedron
cube
tetragonal pyramid
trigonal bipyramid
octahedron square bipyramid
square
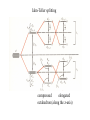
a)
UV/VIS spectra of three
chromium(III) complexes:
a) [Cr(en)3]3+
b) [Cr(ox)3]3c) [CrF6]3look for the shift of the two
b)
absorption peaks 1 and 2 to
lower frequencies.
c)
Spectrochemical series
phosph: 4-methyl-2,6,7-trioxa-1-phosphabicyclo[2.2.2]octane
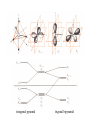
Jahn-Teller splitting
compressed
elongated
octahedron (along the z-axis)
Isomerism in co-ordination compounds
if two or more molecules or ions have the same molecular formula
but the atoms are arranged differently we call them isomers.
The structures of isomers are not superimposable.
Isomers have different physical and/or chemical properties.
We distiguish between
•structural isomers which contain the same number and kind of
atoms, but the connectivity between the atoms is different and
•Stereoisomers which contain both the same number and kind of
atoms and the same connectivity between the atoms but the spatial
arrangement of the atoms is different
Structural isomers
• Ionization isomerism
complex salts which show ionization isomerism
are composed in such a way that a ligand and a
counter ion change their places
[CoCl(NH3)5]SO4
[CoSO4(NH3)5]Cl
Structural isomers
• hydrate isomerism
this a special case of the ionization isomerism. Here
water molecules are present as ligand in one case
and as water of crystallzation in the second case
[Cr(H2O)6]Cl3
[CrCl(H2O)5]Cl2.H2O
[CrCl2(H2O)6]Cl.2H2O
Structural isomers
• Co-ordination isomerism
if in a complex salt both anion and cation are complexes
there can be an exchange of ligands between cation and
anion
[Co(NH3)6] [Cr(CN)6]
[Cr(NH3)6] [Co(CN)6]
Structural isomers
• Linkage isomerism
if a ligand containes more than one atom with a free
electron pair, the ligand may be bound to the central
atom via the different atoms.
C
N
S C
N
N
O
O
bonding via C
bonding via N
cyanoisocyano-
bonding via S
bonding via N
thiocyanatoisothiocyanato-
bonding via N
bonding via O
nitronitrito-
Stereoisomers can be divided in two groups:
•
Enatiomers, i.e. stereoisomers that have a non-superimposable
mirror image
•
Diastereoisomers, i.e. all stereoisomers that are not
enantiomers
Diastereoisomers
• cis - trans isomerism
if a square planar or an octahedral complex containes two
ligands of the same type, they can be arranged so that the angle
L - Z - L is 90° (cis) or 180° (trans)
square planar
octahedral
Cl
Cl
Cl
Pt NH3
NH3
cis
Cl
NH3
Pt NH3
Cl
Co
Cl
Co
Cl
Cl
trans
cis
trans
Diastereoisomers
• fac - mer isomerism
if an octahedral complex containes three ligands of the same
type they can be arranged such that they all are in a cis
position (fac) or that two of them are in a trans position (mer)
Cl
Co
Cl
Cl
Co
Cl
Cl
Cl
fac(ial)
mer(idional)
Enantiomers
• stereoisomers that have a non-superimposable
mirror image are called enantiomers
mirror plane
Co
Cl
Cl
Cl
Cl
Co
The corresponding trans complex is not an enantiomer
mirror plane
Cl
Cl
Co
Co
Cl
Cl
If a molecule or complex is either
asymmetric, i.e. has no symmetry at all, or
dissymmetric, i.e. has no center of inversion or mirror
plane or other Sn,
it is called chiral.
Due to the chirality it has a non-superimposable mirror
image
Optical isomerism
•
if the lifetimes of the two enantiomers of a chiral
molecule are long enough to be separable they are called
optical isomers
•
pure enantiomers are optically active, they rotate the
plane of polarized light in different directions. This is the
only difference in the physical properties of the two
enantiomers
Geometry of complexes
The main structural characteristics of complexes are their co-ordination numbers and their
co-ordination polyhedra.
1. Co-ordination number 2
L
Z
L
Complexes with co-ordination number 2 are rare. They are only formed by central atoms
of the group Cu+, Ag+ and Au+.
The complexes are linear. Bent geometries as they are found in three-atomic molecules
like H2O have never been seen with complexes.
2. Co-ordination number 3
Complexes with co-ordination number 3 are seldom.
Examples are HgI3-, [Pt(P{C6H5}3]3.
L
The complexes are trigonal planar, sometimes
slightly deformed. There is no possibility for the
formation of isomers in complexes of type [ZL2L’] or
[ZLL’L’’]
Some complexes of CN 3 have the form of a trigonal
pyramid like NH3, OR3+ or SR3+ due to a free electron
pair. They are said to be pseudo-tetrahedral as the free
electron pair and the three ligands occupy the four
corners of a tetrahedron.
L
Z
L
Z
L
L
L
3. Co-ordination number 4
For the co-ordination number 4 which is very common there are 4 different
structures possible:
L
L
Z
L
tetrahedral
L
L
L
L
Z
L
L
square planar
Z
L
L
L
bisphenoidal
L
L
Z
L
L
tetragonal pyramid
Examples:
tetrahedral: [Al(OH)4]-, [Cd(CN)4]2-, [BF4]square planar: [PtCl4]2-, [Ni(diacetyldioxim)2], [AuF4]bisphenoidal: main group elements with a free electron pair like As or Sb [AsF 4][SbCl4]there is the possibility that the bisphenoid becomes distorted towards a tetragonal
pyramid when the electron pair needs more space
Sometimes there is a CN of 4 though the formula suggests CN 3
for instance gaseous AlCl3 is dimeric built from two tetrahedra scharing one edge
so that two chloro ligands are bridging and four are end standing
Cl
Cl Al
Cl
Cl
Cl
Al
Cl
Or in the case of (AuCl3)2 the central atoms are square planar co-ordinated
by 4 chloro ligands with 2 of them in bridging positions
Cl
Cl
Cl
Cl
Au
Cl
Au
Cl
4. Co-ordination number 5
this co-ordination number is formed not very often. There are two different
geometries possible:
L
L
Z
L
L
L
L
trigonal bipyramid
L
L
Z
L
L
tetragonal pyramid
In the trigonal bipyramid we can distinguish between equatorial
and apical positions of the ligands
Examples:
trigonal bipyramid: Fe(CO)5, [SnCl5]tetragonal pyramid: [VO(acetylacetonate)2]
5. Co-ordination number 6
of the possible co-ordination geometries
(octahedron, trigonal prismatic, trigonal
antiprismatic and hexagonal planar) only the
octahedron and the trigonal antiprismatic coordination is observed in co-ordination compounds.
Very often the octahedra are not ideal as not all
edges are equally long.
This may be caused by an elongation or a
compression along the 4 fold axis
or
by an elongation along the 3 fold axis leading to the
trigonal antiprismatic polyhedron
C4 axis
L
C3 axis
L
L
Z
L
L
L
Slight deformations of the trigonal bipyramid in the indicated way lead to the
formation of the tetragonal pyramid
A
L
Z
A
L
L
L
L
A
Z
L
L
A
L
Z
A
A
L
This can lead to an exchange of the apical and equatorial positions of the ligands
6. Co-ordination number 7
3 different co-ordination polyhedra exist for CN 7. The energetic difference between
them is low. Sometimes the co-ordination polyhedron changes when the cation changes
L
L
L
L
pentagonal bipyramid
L
L
L
L
L
L
L
L
Z
L
L
L
L
monocapped trigonal prism
Examples:
pentagonal bipyramid: [UO2F5]3-, [HfF7]3moncapped trigonal prism: [TaF7]3monocapped octahedron: [IF6]-, [NbOF6]3-
L
L
Z
L
L
L
monocapped octahedron
7. Co-ordination number 8
4 different co-ordination polyhedra exist for CN 8. The energetic differences between
them are low. They become lower with increasing CN.
cube
Z
square antiprism
Z
dodecahedron
Examples:
cube: seldom, but [UF8]3square antiprism: more stable than cube [TaF8]3- , [ReF8]3dodecahedron: [Mo(CN)8]4- , [W(CN)8]4hexagonal bipyramid: [UO2(acetylacetonate)3]-
Z
Z
hexagonal bipyramid