* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Acids and Bases
Gaseous signaling molecules wikipedia , lookup
Cell-penetrating peptide wikipedia , lookup
Genetic code wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Biosequestration wikipedia , lookup
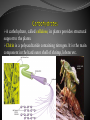
List of types of proteins wikipedia , lookup
Expanded genetic code wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Lecture 6 Acids and Bases & Organic Chemistry Ozgur Unal 1 Remember the polarity of water that can dissolve sugar and salt. A substance that contain H might release a hdrogen ion (H+) because it is attracted to the Oxygen in water. These substances are called acids. Example: Hydrochloric acid (HCl) Substances that release hydroxide ion (OH-) when dissolved in water are called bases. Example: Sodium hydroxide (NaOH) 2 The concentration of H+ or OH+ determines the strength of an acid or a base. The measure of concentration of these ions in a solution is called pH. pH values lower than 7 indicates acids, whereas values higher than 7 indicates bases. Water has a pH of 7. 3 The majority of biological processes carried out by cells occure between pH 6.5 and 7.5. In order to maintain homeostasis, it is important to control this pH level. Buffers are mixtures that can react wit acids or bases to keep the pH within a particular range. Your blood contains buffers that keep the pH about 7.4 http://www.mhhe.com/physsci/chemistry/essential chemistry/flash/buffer12.swf 4 Almost all biological molecules contain the element Carbon ------- > life on Earth is carbon-based. Organic chemistry is the study of organic compounds – compounds that contain carbon. Why is carbon so special? Carbon has 4 electrons in its outermost shell, therefore it can form 4 covalent bonds. Carbon atoms can bond to each other. There is a variety of important organic compounds that can be formed thanks to carbon. 5 Carbon atoms can be joined to form carbon molecules. Cells store smaller carbon compounds to build larger molecules. Macromolecules are large molecules that are formed by joining smaller organic molecules together. Biological macromolecules are organized into four major categories: 1) Carbohydrates 2) Lipids 3) Proteins 4) Nucleic Acids 6 Lecture 7 Biological Macromolecules Ozgur Unal 7 Carbohydrates are composed of hydrogen, oxygen and carbon in a fixed ratio. For every carbon atom , there is one oxygen atom and two hydrogen atoms. The general formula for carbohydrates is written as (CH2O)n. If the value of n ranges from 3 to 7, the molecules are called sugar or monosaccharides. Monosaccharides can be linked to form larger molecules (such as disaccharide and polysaccharide). Check Figure 6.26! 8 Glucose is a monosaccharide Sucrose (table sugar) and Lactose (in milk) are disaccharide Glycogen is a polysaccharide Carbohydrates are used as energy stores in a living body. Excess glucose is stored as glycogen in the body. When the body needs energy between meals, glycogen is broken down into glucose. 9 A carbohydrate, called cellulose, in plants provides structural support to the plants. Chitin is a polysaccharide containing nitrogen. It is the main component in the hard outer shell of shrimp, lobster etc. 10 Lipids are molecules made mostly of carbon and hydrogen that make up the fats, oils and waxes. The primary function of lipids is to store energy. A lipid called triglyceride is a fat if it is solid at room temperature, an oil if it is liquid at room temperature. Plant leaves are coated with lipids called waxes to prevent water loss. A honeycomb in a beehive is made of bees wax. 11 The basic structure of a lipid includes fatty acid tails. Saturated fats have only single bonds between the carbon atoms, therefore no hydrogen atom can bond to the tail. Unsaturated fats have at least one double bond between carbon atoms, therefore the tail can accomodate at least one more hydrogen. 12 Phospholipids are responsible for the structure and function of the cell membrane. Phospholipids are hydrophobic (meaning they do not dissolve in water). They act as barriers in biological membranes. Steroids are a type of lipid that include substances such as hormones and cholesterol 13 Proteins make up 15% of your total mass and are involved in every function in your body. 10,000 different proteins Structural support Transport substances Speed up chemical reactions etc. 14 A protein is a compound made of small carbon compounds called amino acids. Amino acids are small compounds that are made of carbon, oxygen, hydrogen and sometimes sulfur. All amino acids share the same general structure. Central carbon Hydrogen Amino group (H2N) Carboxyl group (COOH) Variable (R) There are 20 different variable group. 15 Amino acids are joined together to form proteins by covalent bonds called peptide bonds. A peptide forms between the amino group of one amino acid and the carboxyl group of another. 16 Proteins can have up to four levels of structure. 1- Primary structure: The number and order of aminoacids 2- Secondary structure: Sequence of amino acids linked by a hydrogen bond 3- Tertiary structure: Globular structure 4- Quarternary structure: Combining with other proteins 17 Nucleic acids are complex macromolecules that store and transmit genetic information. Nucleid acids are made of subunits called nucleotides. Nucleotides are composed of carbon, nitrogen, oxygen, phosphorus and hydrogen atoms. Three units: Phosphate Nitrogenous base Ribose sugar 18 Two types of nucleic acids found in living organisms. DNA: Deoxyribonucleic aicd RNA: Ribonucleic acid In DNA and RNA, sugar of one nucleotide bonds to the phosphate of another nucleotide. (Check Figure 6.31) A nucleotide with 3 phosphate groups is adenosine triphosphate, ATP. ATP is used to store chemical energy that can be used by cells. 19