* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download File
Ultraviolet–visible spectroscopy wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Acid–base reaction wikipedia , lookup
Homoaromaticity wikipedia , lookup
Ionic compound wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Equilibrium chemistry wikipedia , lookup
State of matter wikipedia , lookup
Spinodal decomposition wikipedia , lookup
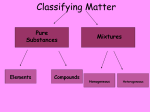
Matter Matter is anything that has volume & mass (including gasses). Things that are not made of matter include fire, light, gravity, sound, and heat. All matter is classified as a pure substance or a mixture based on its composition. What qualifies something as a pure substance? A pure substance has a fixed composition. Pure substances are made of a single repeating particleeither an element or a molecule- with nothing else mixed in. Pure substances are either compounds or elements. They cannot be separated into its components by any physical processes What Classifies something as an element? An element is a pure substance that cannot be broken down into simpler, stable substances and is made of one type of atom. There are over 100 elements, all listed on the periodic table. An atom is the smallest unit of an element that maintains the chemical identity of that element. What classifies something as a compound? A compound is a substance that can be broken down into simple stable substances through chemical reactions. A molecule is the smallest unit of an compound made from two or more bonded atoms in a fixed composition. The composition of a compound is represented by a chemical formula. Compounds are held together by chemical bonds How are elements different from compounds? Elements are composed of only one type of atom Ex. Si, O2 They usually do not have a chemical bond, unless the element is diatomic, which means it only exists in nature as a pair Compounds are composed of more than one type of atom Ex. SiO2 They must have at least one chemical bond How do the properties of elements change when they become compounds? The properties of compounds are quite different from those of their component elements. When the elements sodium and chlorine combine chemically to form sodium chloride, there is a change in composition and a change in properties. Sodium is a soft gray metal that is explosive when placed in water Chlorine is a pale yellow poisonous gas. Sodium chloride (commonly known as table salt) is a white solid. What qualifies something as a mixture? A mixture is a blend of two or more kinds of matter, each of which retains its own identity and properties. mixed together physically can usually be separated physically composition is not uniform has no chemical formula Types of Mixtures Homogeneous mixtures are called solutions uniform in composition (salt-water solution) does not look like a mixture Heterogeneous mixtures not uniform throughout (sand-water mixture) the mixtures components are visible Homogeneous vs. Heterogeneous Solution a homogeneous mixture can be physically separated composed of solutes and solvents the substance in the smallest amount and the one that dissolves in the solvent Iced Tea Mix (solute) the substance in the larger amount that dissolves the solute Iced Tea (solution) Water (solvent) Water is considered the universal solvent as it is the solvent used to sustain all life Solutes Change Solvents The amount of solute in a solution determines how much the physical properties of the solvent are changed Examples: Lowering the Freezing Point The freezing point of a liquid solvent decreases when a solute is dissolved in it. Ex. Pure water freezes at 00C, but when salt is dissolved in it, the freezing point is lowered. This is why people use salt to melt ice. Raising the Boiling Point The boiling point of a solution is higher than the boiling point of the solvent. Therefore, a solution can remain a liquid at a higher temperature than its pure solvent. Ex. The boiling point of pure water is 1000C, but when salt is dissolved in it, the boiling point is higher. This is why it takes salt water longer to boil than fresh water. Concentration the amount of solute dissolved in a solvent at a given temperature •described as dilute if it has a low concentration of solute •described as saturated if it has a high concentration of solute •described as supersaturated if contains more dissolved solute than normally possible Solubility The ability of a solute to be able to dissolve in a solution The more soluble a substance is, the greater the concentration the solution is capable of having Influenced by: Temperature Pressure Solids increased temperature causes them to be more soluble and vice versa Solids increased pressure has no effect on solubility Gases increased temperature causes them to be less soluble and vice versa Gases increased pressure causes them to be more soluble and vice versa Ex. Iced Coffee Ex. Soda, “The Bends”