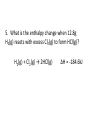* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Ch 10 Review activity
Radiator (engine cooling) wikipedia , lookup
Dynamic insulation wikipedia , lookup
Hypothermia wikipedia , lookup
Heat exchanger wikipedia , lookup
Cogeneration wikipedia , lookup
Solar water heating wikipedia , lookup
Copper in heat exchangers wikipedia , lookup
Solar air conditioning wikipedia , lookup
R-value (insulation) wikipedia , lookup
Heat equation wikipedia , lookup
Intercooler wikipedia , lookup
Thermal conduction wikipedia , lookup
1. A 322 g sample of lead (specific heat = 0.138 J/goC) is placed into 264 g of water at 25oC. If the system's final temperature is 46oC, what was the initial temperature of the lead? 2. A 97 g sample of gold at 785oC is dropped into 323 g of water, which has an initial temperature of 15oC. If gold and water have specific heats of 0.129 J/goC and 4.184 J/goC respectively, what is the final temperature of the mixture? Assume that the gold and the water experience no change in state of matter 4Fe(s) + 3O2(g) → 2Fe2O3(s) ΔH = -1652 kJ 3. How many kcal are released when 1.00g of Fe is reacted with excess O2? 4. Calculate the heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67.4 kJ of heat, and its temperature changes from 32°C to 57°C. When this piece of wood is burned, would the combustion reaction be endothermic or exothermic? Where does the energy that would be transferred in this reaction come from? 5. What is the enthalpy change when 12.8g H2(g) reacts with excess Cl2(g) to form HCl(g)? H2(g) + Cl2(g) → 2HCl(g) ΔH = -184.6kJ 6. How much heat, in calories and kilocalories, does it take to raise the temperature of 814g of water from 18.0°C to 100°C? 7. What mass of water, in kilograms, can be heated from 5.5°C to 55.0°C by 9.09x1010J of heat? 8. A 0.8082g sample of glucose (C6H12O6) is burned in a bomb calorimeter assembly, and the temperature is noted to rise from 25.11°C to 27.21°C. Determine the heat capacity of the bomb calorimeter assembly, given: C6H12O6(s) + 6O2(g) 6CO2(g) + 6H2O(l) ΔH = -2803kJ/mol 9. If 40.5 J of heat is added to a 15.4 g sample of silver, how much will the temperature increase by? The specific heat of silver is 0.235 J/goC. 10. What is the specific heat of silicon if the temperature of a 4.11 g sample of silicon is increased by 3.8 oC when 11.1 J of heat is added? 11. Convert 23 calories into kcal. Convert 7.9 kcal into calories. Convert 234 J into kJ. Convert 21.3 kJ into joules. Convert 58 J into calories. Convert 34 kJ into kcal. 1. Convert 23 calories into kcal. 2. Convert 7.9 kcal into calories. 3. Convert 234 J into kJ. 4. Convert 21.3 kJ into joules. 5. Convert 58 J into calories. 6.Convert 34 kJ into kcal.