* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 2.5 Chemical Bonding - Lighthouse Christian Academy
Survey
Document related concepts
Cluster chemistry wikipedia , lookup
Metastable inner-shell molecular state wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Physical organic chemistry wikipedia , lookup
State of matter wikipedia , lookup
Aromaticity wikipedia , lookup
Molecular orbital wikipedia , lookup
Electron configuration wikipedia , lookup
Homoaromaticity wikipedia , lookup
Atomic theory wikipedia , lookup
Nanofluidic circuitry wikipedia , lookup
Ionic liquid wikipedia , lookup
Transcript
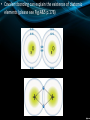
Chemical Bonding (Sec 7.2 pg 176 – 181) • The physical forces that join or connect atoms together are called chemical bonds. • Remember: BONDING involves interactions b/w the ELECTRONS of 2 or more atoms. • There are 2 general types of bonding (ionic and covalent): • Oppositely charged ions (metals and non-metals) have a strong attraction for one another and, as a result, are held tightly together. • This is known as ionic bonding and serves to build atoms into compounds called ionic compounds. • In ionic bonding, a transfer of valence e occurs. • Ionic bonding always occurs b/w metals and nonmetals • Some properties of ionic bonds: – Form crystals (See Fig.2 p.177) – Have high melting points (LOTS heat needed to separate tightly bound ions) – Hard and brittle – Conduct electricity when dissolved in water. • Another type of bonding occurs when non-metals ‘share’ their valence e with other non-metals to complete their valence shells. • This bonding is called covalent bonding and builds atoms into covalent or molecular compounds. • Therefore, in covalent bonding, a sharing of valence electrons occurs (always b/w 2 non-metals). • Some properties of covalent bonds: – Form crystals – Do not conduct electricity when dissolved (please see Fig.9 p.181) – Have low melting and boiling points. • Covalent bonding can explain the existence of diatomic elements (please see Fig.4&5 p.178) • The third kind of bond is a cross between the ionic bond and the covalent bond. • It is called the polar covalent bond and it forms when the two elements share valence electrons but one element pulls the electrons closer to its nucleus. • The diagram on the right shows the breakdown of pure substances: • Atoms are the smallest unit of elements (in chemistry…) • Ions are the smallest unit for ionic compounds. • Molecules are neutral particles that consist of 2 or more atoms covalently bonded together (Fig.6 p.178) – they form molecular compounds Check your Understanding Pg 182 #1-5, 7, 10