* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Full Text
Cardiovascular disease wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Cardiothoracic surgery wikipedia , lookup
Marfan syndrome wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
History of invasive and interventional cardiology wikipedia , lookup
Myocardial infarction wikipedia , lookup
Turner syndrome wikipedia , lookup
Cardiac surgery wikipedia , lookup
Coronary artery disease wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Aortic stenosis wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
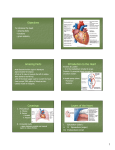
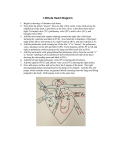
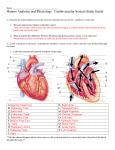
Journal of the American College of Cardiology © 2006 by the American College of Cardiology Foundation Published by Elsevier Inc. EDITORIAL COMMENT Aortic Regurgitation After Arterial Switch Operation* Pedro J. del Nido, MD, FACC,† Marcy L. Schwartz, MD‡ Boston, Massachusetts In the early 1980s, Jatene developed the arterial switch operation (ASO), which soon became the preferred surgery for d-loop transposition of the great arteries (TGA). This operation includes translocation of the pulmonary artery and aorta above the sinuses, often with a Lecompte maneuver (bringing the pulmonary artery anterior to the aorta) and coronary reimplantation into the neo-aortic sinuses. Although the complications inherent in the previous atrial switch procedures were avoided, and the left ventricle could remain the systemic ventricle, other post-operative complications following ASO have been encountered. With increased surgical experience, the initial problems seen with coronary transfer are now rare. Unusual coronary patterns, once considered to be a risk factor for survival, have been found more recently not to affect outcome (1). Supravalvar pulmonary stenosis at the reanastomosis site or branch pulmonary stenosis due to tension has been observed. At short- and longer-term follow-up, neo-aortic root dilation and neo-aortic regurgitation (AR) are relatively common, infrequently leading to reoperation. See page 2057 In this issue of the Journal, Losay et al. (2) report the incidence and risk factors of aortic regurgitation in a very large cohort of 1,156 patients at long-term follow-up after ASO. Although aortic regurgitation is relatively common, approximately 15% at a mean follow-up of 76 months and up to 30% by 15 years, the majority of patients had only trivial or mild regurgitation, and higher degrees of regurgitation (2.1%) and AR requiring reoperation (1.4%) were rare. Furthermore, the authors also report that although the prevalence of AR increases over time following ASO, the degree of AR may actually decrease during follow-up. Since trivial or mild native pulmonary regurgitation can be considered to be normal, the relevant issue may not be the overall incidence of AR, but instead the incidence and risk factors of significant AR necessitating close follow-up and *Editorials published in the Journal of the American College of Cardiology reflect the views of the authors and do not necessarily represent the views of JACC or the American College of Cardiology. From the †Department of Cardiac Surgery, Department of Surgery, and the ‡Department of Cardiology, Department of Pediatrics, Children’s Hospital, Harvard Medical School, Boston, Massachusetts. Vol. 47, No. 10, 2006 ISSN 0735-1097/06/$32.00 doi:10.1016/j.jacc.2006.02.026 sometimes aortic valve repair or replacement. As acknowledged by the authors, a limitation of this study is that AR quantification was not defined or standardized among the several institutions providing follow-up assessment and classification. The authors of this study found that by univariate analysis, AR of all degrees was associated with pre-operative aorta-pulmonary artery size discrepancy, pulmonary artery band placement, aortic arch obstruction, and older age at ASO. By multivariate analysis, risk factors for AR were regurgitation at hospital discharge after ASO and complex TGA, defined as presence of a surgical ventricular septal defect (VSD) or Taussig-Bing anomaly. In the present study, risk factors for more significant AR and/or for aortic valve surgery were not reported. From their findings, the authors of this study speculate that AR is related to high pressure and flow in the pulmonary artery before ASO. Surgical technique, sometimes including VSD repair, may also be a risk factor. Other potential explanations for AR after ASO should be considered. Several studies have also shown that pulmonary artery banding before ASO is related to the development of AR (3–5). In a recent study, the only risk factor for at least moderate AR by multivariate analysis was age ⬎1 year at ASO, which was closely related to VSD repair at the time of ASO and a previous pulmonary artery band (6). In that study, it was not possible to distinguish which was the most important risk factor because of the high covariance of these variables, and VSD repair at the time of ASO was the only independent risk factor for subsequent neo-aortic valve or root repair. In one study, AR was not as commonly seen when the indication for pre-operative pulmonary artery band was to restrict pulmonary blood flow as when following a two-stage ASO. This suggested that the etiology of AR was not only pulmonary artery distortion, but possibly also the rapid rise in pressure encountered by an unprepared pulmonary valve (4). Risk factors for AR have been reported to overlap with those of aortic root dilation. A correlation between degree of AR and aortic root dilation has been described, but significant overlap was seen between groups (6). Measurement of aortic root dimension was not performed in the present study, and therefore the role of root dilation in the development of AR was not investigated. Looking more closely at the time of onset and rate of progression of significant regurgitation in the series by Losay et al. (2), a familiar pattern is discernable. Excluding the group that had AR shortly after ASO, there is a gradual decline in the number that are free from AR and reintervention over the 10 to 15 years of follow-up. This curve is remarkably similar to the experience with the Ross operation, where the pulmonary root is inserted into the left ventricular outflow as a root replacement. With late follow-up of the Ross procedure in children and young 2064 del Nido and Schwartz Editorial Comment adults now exceeding 10 years, the incidence of aortic root dilatation (Z-score ⬎3) is very high (90% in some series), and freedom from aortic valve regurgitation and need for reoperation falls to approximately 70% by 10 years (7,8). Reoperation for AR was less common in the series described by Losay et al. (2); however, the rise in the late hazard function is similar to the Ross experience. The causes of AR after ASO are clearly multi-factorial, possibly including tissue characteristics of the native pulmonary wall and valve, as well as pre-operative and operative risk factors. Histologic examination of the pulmonary root has shown important differences compared to the aorta with respect to elastin layer and smooth muscle actin, even in transposition (9). Although long-term results of the ASO are encouraging, long-term surveillance for aortic root dilatation and AR remains necessary. Reprint requests and correspondence: Dr. Pedro J. del Nido, Department of Cardiac Surgery, Children’s Hospital, 300 Longwood Avenue, Boston, Massachusetts 02115. E-mail: Pedro. [email protected]. JACC Vol. 47, No. 10, 2006 May 16, 2006:2063–4 REFERENCES 1. Blume ED, Altmann K, Mayer JE, et al. Evolution of risk factors influencing early mortality of the arterial switch operation. J Am Coll Cardiol 1999;33:1702–9. 2. Losay J, Touchot A, Capderou A, et al. Aortic valve regurgitation after arterial switch operation for transposition of the great arteries: incidence, risk factors, and outcome. J Am Coll Cardiol 2006;47:2057– 62. 3. Di Donato RM, Wernovsky G, Walsh EP, et al. Results of the arterial switch operation for transposition of the great arteries with ventricular septal defect. Circulation 1989;80:1689 –705. 4. Jenkins KJ, Hanley FL, Colan SD, et al. Function of the anatomic pulmonary valve in the systemic circulation. Circulation 1991;84: III173–9. 5. Martin RP, Ettedgui JA, Qureshi SA, et al. A quantitative evaluation of aortic regurgitation after anatomic correlation of transposition of the great arteries. J Am Coll Cardiol 1988;12:1281– 4. 6. Schwartz ML, Gauvreau K, del Nido P, et al. Long-term predictors of aortic root dilation and aortic regurgitation after arterial switch operation. Circulation 2004;110 Suppl II:128 –32. 7. Luciani GB, Favaro A, Casali G, Santini F, Mazzucco A. Ross operation in the young: a ten-year experience. Ann Thorac Surg 2005; 80:2271–7. 8. Hazekamp MG, Grotenhuis HB, Schoof PH, Rijlaarsdam ME, Ottenkamp J, Dion RA. Results of the Ross operation in a pediatric population. Eur J Cardiothorac Surg 2005;27:975–9. 9. Lalezari S, Hazekamp MG, Bartelings MM, Schoof PH, Gittenberger-De Groot AC. Pulmonary artery remodeling in transposition of the great arteries: relevance for neoaortic root dilatation. J Thorac Cardiovasc Surg 2003;126:1053– 60.