* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download CH 2
Survey
Document related concepts
Transcript
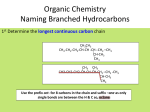
Simple Organic Chemistry Basic Structure and Nomenclature Adapted from http://www.sciencegeek.net/APchemistry/Powerpoints.shtml First Ten Alkanes Formula Name Formula Name CH4 Methane C6H14 Hexane C2H6 Ethane C7H16 Heptane C3H8 Propane C8H18 Octane C4H10 Butane C9H20 Nonane C5H12 Pentane C10H22 Decane Alkane = CnH2n+2 Condensed Structural Formula = CH3(CH2)nCH3 Straight Chain Alkanes aren’t “Straight” C – C bonds are sp3 hybridized CH3 H3C Butane, C4H10 Decane, C10H22 Rules for Naming Alkanes (Nomenclature) 1. For a branched hydrocarbon, the longest continuous chain of carbon atoms gives the root name for the hydrocarbon H3C1 2 H3C 3 4 CH3 4 carbon chain = butane Rules for Naming Alkanes (Nomenclature) 2. When alkane groups appear as substituents, they are named by dropping the -ane and adding -yl. —CH3 Methyl —CH2CH3 Ethyl —CH2CH2CH3 Propyl —CH2CH2CH2CH3 Butyl H3C H3C Methyl CH3 Rules for Naming Alkanes (Nomenclature) 3. The positions of substituent groups are specified by numbering the longest chain of carbon atoms sequentially, starting at the end closest to the branching. H3C1 2 3 H3C Methyl 4 CH3 Rules for Naming Alkanes (Nomenclature) 4. The location and name of each substituent are followed by the root alkane name. The substituents are listed in alphabetical order (irrespective of any prefix), and the prefixes di-, tri-, etc. are used to indicate multiple identical substituents. H3C1 2 3 H3C Methyl 4 CH3 Name: 2-methylbutane Nomenclature Practice Name this compound CH3 H3C1 2 Cl 3 4 5 CH3 6 7 9 carbons = nonane 8 H3C9 Step #1: For a branched hydrocarbon, the longest continuous chain of carbon atoms gives the root name for the hydrocarbon Nomenclature Practice Name this compound CH3 H3C1 2 Cl 3 4 5 CH3 6 7 8 9 carbons = nonane CH3 = methyl chlorine = chloro H3C9 Step #2: When alkane groups appear as substituents, they are named by dropping the -ane and adding -yl. Nomenclature Practice Name this compound CH3 H3C1 2 Cl 3 4 5 6 7 9 carbons = nonane CH3 CH3 = methyl chlorine = chloro 8 H3C9 1 9 NOT 9 1 Step #3: The positions of substituent groups are specified by numbering the longest chain of carbon atoms sequentially, starting at the end closest to the branching. Nomenclature Practice Name this compound CH3 H3C1 2 Cl 3 4 5 CH3 6 7 9 carbons = nonane CH3 = methyl 8 chlorine = chloro H3C9 2-chloro-3,6-dimethylnonane Step #4: The location and name of each substituent are followed by the root alkane name. The substituents are listed in alphabetical order (regardless of any prefix), and the prefixes di-, tri-, etc. are used to indicate multiple identical substituents. Cyclic Alkanes Cyclopropane, C3H6 Cyclobutane, C4H8 Cyclopentane, C5H10 Cyclohexane, C6H12 Cycloheptane, C7H14 Remember, explicit hydrogens are left out Structural Isomers Isomers are molecules with the same chemical formula, but different organization of atoms (different bonding) n-Pentane, C5H12 2-methylbutane, C5H12 H3C CH3 H3C CH3 CH3 2,2-dimethylpropane, C5H12 CH3 H3C CH3 CH3 Alkenes Contain Carbon-Carbon Double bonds 1 bond H H C H H C Each carbon is sp2 hybridized H C H H C H Ethene 1 bond Alkenes are considered unsaturated Geometric Isomerism in Alkenes • Unhybridized p orbitals must align, so bond cannot rotate cis-2-butene trans-2-butene Alkynes Contain Carbon-Carbon Triple Bonds H H C C C H 1 bond C Each carbon is sp hybridized Ethyne 1 bond 1 bond Alkynes are considered unsaturated H Reactions of Alkenes and Alkynes Hydrogenation CH 2 CHCH 3 H 2 CH 3 CH 2 CH 3 Propene Propane Catalyst Dehydrogenation Ethane Ethene Polymerization Small molecules are joined together to form a large molecule CH2 CH2 Polyethylene n Reactions of Alkenes and Alkynes Combustion Propane Halogenation CH 2 CHCH 2 CH 2 CH 3 Br2 CH 2 BrCHBrCH 2 CH 2 CH 3 1-Pentene 1-2-dibromopentene Substitution Aromatic Hydrocarbons Cyclic unsaturated hydrocarbons with delocalized electrons The simplest aromatic hydrocarbon is benzene (C6H6) H H H H H H H H H H H OR… H H H H H H H Geometric Isomerism in Aromatics ortho (o-) = two adjacent substituents o-dichlorobenzene Cl H Cl H H H meta (m-) = one carbon between substituents m-dichlorobenzene para (p-) = two carbons between substituents p-dichlorobenzene Cl H H Cl H H Cl H H H H Cl Hydrocarbon Derivatives Class Alcohol Functional Group hydroxyl group General Formula -O — H R – OH Alkyl halide —X R—X Ether —O— R — O — R’ Aldehyde carbonyl group O || —C—H O || R—C—H Ketone carbonyl group O || —C— O || R — C — R’ Carboxylic acid carboxyl group O || — C — OH Ester Amine amine group O || — C — OH O || — C — O— O || R — C — O — R’ | —N— R’ | R — N — R’’ Alcohols • -OH group • Higher than expected boiling point (based on molecular weight) • Naming is based on longest carbon chain attached to –OH group – End in -ol Aldehydes & Ketones -al -one • No numbers used for naming aldehydes since group is always on terminal carbon Carboxylic Acids & Esters • Carboxylic acids are weak acids – Examples: acetic, salicylic • Esters have a fragrant, often pleasant odor • Esterification: Amines (-NH2) • Derivatives of ammonia where one or more N-H bond is replaced by N-C bond • Figure 22.18 shows the structures of the 20 amino acids