* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download VEGF exists in different isoforms
Survey
Document related concepts
Transcript
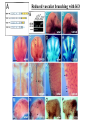
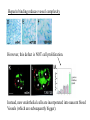
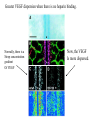
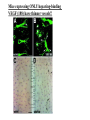
The ECM as a Spatial Organizer of Growth Factors Chris Liu 8/21/03 What happens when growth factors are secreted/released extracellular? What is a Morphogen? 1) Long-range signalling molecule 2) Acts directly on cells (Wolpert, 1969) Different models of Morphogen Action a) Morphogen Diffusion b) Morphogen inhibitor diffusion c) Cytoneme function a) Close physical interaction b) Cytoplasmic diffusion. Along limb buds, can see morphogen gradients. These factors may be dependent upon: morphogen transport morphogen stability There is also a lot of regulation of morphogen receptors. Are there other factors? Heparin Sulfate Proteoglycan (HSPGs) localize many morphogens. Some representative HSPGs These are characterized as “cofactors” for signalling. Through genetic studies, they modify the phenotypes GFs and GFRs Heparin synthesis genes have also been implicated. VEGF exists in different isoforms heparin binding common none rare common 50-70% common rare total VEGF exists in different isoforms common rare common Releases soluble VEGF common rare Cellular Receptors for VEGF There appears to be synergy between NRP1 and VEGFR-2 VEGF-A (through KO studies) is essential for : embryonic blood vessel formation, remodeling, and survival. embryonic lethal. The same is true for VEGF-Rs (Flk1 and Flt1) wtVEGF-/- but VEGF120+/+ have: normal embryonic vascular development widespread vessel patterning defect fewer branch points. larger luminal diameter. (Carmeliet 1999, Stalmans and Ng 2002) post-natal angiogenesis defects. (Shima group) Reduced vascular branching with KO Heparin binding reduces vessel complexity However, this defect is NOT cell proliferation. Instead, new endothelial cells are incorporated into nascent blood Vessels (which are subsequently bigger). Greater VEGF dispersion when there is no heparin binding. Normally, there is a Steep concentration gradient Of VEGF Now, the VEGF Is more dispersed. Mice expressing ONLY heparing-binding VEGF (188) have thinner vessels!! Too much or too little heparin binding of VEGF is a problem However, double heterozygotes compensate for each other and are wt. Various members of the VEGF/PDGF and bFGF are alternatively spliced proteolyzed. which regulate proteoglycan and ECM binding. Ref (Betsholtz, 2001, Eriksson and Alitalo 1999) (Sunny?) Are there other molecules that regulate VEGF/growth factors? Fibronectin binds VEGF VEGF and FN induce migration. VEGF and FN do not induce Migration alone (Wijelath, …Rafii, Sobel, Circ Res) VEGF/FN mediate alpha5beta/FLK-1 interaction Anti-a5b1 inhibits migration FN contains 2 VEGF binding sites FN can be proteolyzed into fragments VEGF binds mainly FN Only the N- and C-terminal (not RGD) fragments bind VEGF Model of VEGF/FN/Flk-1/a5b1 action Complex induces downstream signalling. Any thoughts? How important is this microenvironment? (Not at cellular level, not at organismal level, but somewhere in between) How important is release? MMPs reveal cryptic binding sites. Proteases may release bioavailability.