* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download West Nile Virus (WNV) Infection - Health Protection Surveillance
Orthohantavirus wikipedia , lookup
Herpes simplex virus wikipedia , lookup
Meningococcal disease wikipedia , lookup
Ebola virus disease wikipedia , lookup
Rocky Mountain spotted fever wikipedia , lookup
Gastroenteritis wikipedia , lookup
Chagas disease wikipedia , lookup
Dirofilaria immitis wikipedia , lookup
Onchocerciasis wikipedia , lookup
Sexually transmitted infection wikipedia , lookup
Sarcocystis wikipedia , lookup
Neisseria meningitidis wikipedia , lookup
Neonatal infection wikipedia , lookup
Trichinosis wikipedia , lookup
African trypanosomiasis wikipedia , lookup
Eradication of infectious diseases wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
Henipavirus wikipedia , lookup
Hepatitis C wikipedia , lookup
Marburg virus disease wikipedia , lookup
Schistosomiasis wikipedia , lookup
Oesophagostomum wikipedia , lookup
Hepatitis B wikipedia , lookup
Leptospirosis wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
Middle East respiratory syndrome wikipedia , lookup
Coccidioidomycosis wikipedia , lookup
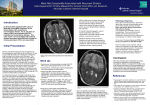
Clinical Information on West Nile Virus (WNV) Infection 2005 Season Introduction and Epidemiology In 1999, West Nile Virus (WNV), an Old World flavivirus, producing a spectrum of disease including severe meningoencephalitis, appeared in North America for the first time. The initial outbreak of WNV infection (centred in New York City) led to 62 cases of meningoencephalitis (59 of them requiring hospitalisation) and seven deaths. Since then, it has spread effectively across the continent, overtaking endemic St Louis encephalitis as the commonest flaviviral CNS infection. Transmission of WNV occurs through the bite of infected mosquitoes that have acquired the virus by feeding on infected birds. In 2003, there was widespread WNV activity over most of the continental United States; a total of 9862 human cases of WNV infection were reported, with 264 deaths. In 2003, Canada reported 1388 human cases, 14 of them fatal. A decline was noted in 2004, with 1142 cases of severe neuroinvasive disease (2539 cases of symptomatic WNV infection in total) leading to 100 deaths in the US. So far in 2005 (1/8/2005), there have been 18 cases of severe neuroinvasive disease and two deaths in the US. The peak season in the US for WNV cases is between mid July and early October. In Europe, there has been sporadic WNV activity in many countries bordering the Mediterranean and in Southeastern Europe over the last 25 years with occasional human cases being diagnosed. In Western Europe during 2003, there was one imported human WNV case in the Netherlands and two cases in France (acquired either locally or in Spain). In 2004, two Irish holidaymakers returning from the Algarve in Portugal were diagnosed as having WNV infection, one with symptoms suggestive of CNS infection. On foot of this development, the European Commission has produced Community-wide case definitions for WNV infection (see page 6). A recent risk assessment in the UK estimated the risk of indigenous WNV infection in the UK and Ireland to be low. Retrospective testing of human cases of encephalitis in 2003 to determine the burden of silent WNV disease including possible UK-acquired disease did not identify any cases. Similarly, testing of mosquitoes from selected wetland areas showed no Health Protection Surveillance Centre WNV - Information for Clinicians – Aug 2005 1 evidence of WNV infection of vectors. Neutralising antibodies to WNV have, however, been identified in certain migrant and resident bird species in the UK. In response to the emergence of WNV in the US (and progressively warmer summers that have the potential to favour the expansion of WNV in Europe), recent UK guidance has been expanded, requesting that clinicians consider testing for WNV in patients with suggestive symptoms, including those without a history of recent travel in North America. The position adopted by the UK is a precautionary one. While recognising that the possibility of WNV becoming established in the UK is low, authorities there consider that the possibility cannot be ruled out. Given that this possibility exists, in Ireland we have, since 2004, requested that clinicians be suspicious of suggestive illness in individuals over the age of 50 irrespective of their travel history but having a high index of suspicion of those with a history of recent travel to North America and to request diagnostic testing accordingly. Non-mosquitoborne WNV Transmission In addition to mosquitoborne spread of WNV, a number of other routes of transmission have been identified. In 2002, transmission of WNV through blood transfusion was first documented in the US. Six probable cases of WNV cases due to transfusion were documented in 2003, and a further case in 2004, indicating that infectious blood components with low concentrations of WNV may escape current screening tests. Also in 2002, intrauterine WNV transmission and WNV transmission through breast milk was also reported. The following clinical information on the cardinal clinical features of WNV infection is intended to provide information for clinicians who encounter a patient with suggestive symptoms. Clinical Features 1. Mild Infection • About 80% of WNV infections are very mild and clinically inapparent. • Few people infected will be able to recall any mosquito bite. • Approximately 20% of those infected develop a mild illness (West Nile fever). • The incubation period is between 3 to 14 days. • Symptoms generally last 3 to 6 days. Health Protection Surveillance Centre WNV - Information for Clinicians – Aug 2005 2 • West Nile Fever is generally described as a febrile illness of sudden onset often accompanied by: a Malaise a Headache a Anorexia a Myalgia a Nausea a Rash a Vomiting a Lymphadenopathy a Eye pain 2. Severe Infection • Approximately 1 in 150 infections will result in severe neurological disease. • Case fatality rates during recent outbreaks have ranged from 4% in Romania (1996) to 12% in New York (1999) and 14% in Israel (2000). • The most significant risk factor for developing severe neurological disease is advanced age (especially over 50; those over 80 are particularly vulnerable). • Encephalitis is more commonly reported than meningitis. • In recent outbreaks, symptoms occurring among patients hospitalised with severe disease include: 9 Fever 9 Headache 9 Weakness 9 Changes in mental status 9 Gastrointestinal symptoms • Almost half of hospitalised cases in the US outbreaks had severe muscle weakness and this may provide a clue to severe WNV infection especially in conjunction with symptoms of encephalopathy. • A minority of patients with severe disease developed a maculopapular or morbilliform rash involving the neck, trunk, arms, or legs. • About 10% of patients demonstrated complete flaccid paralysis. Initially the first cases were though to be suffering from Guillain-Barré syndrome. • • Neurological presentations included: 9 Ataxia and extrapyramidal signs 9 Optic neuritis 9 Cranial nerve abnormalities 9 Polyradiculitis 9 Myelitis 9 Seizures Although not observed in recent outbreaks, myocarditis, pancreatitis, and fulminant hepatitis have been described. • Encephalopathy along with severe muscle weakness, changes in level of consciousness and advanced age were the most powerful clinical predictors of death in those with severe disease. Health Protection Surveillance Centre WNV - Information for Clinicians – Aug 2005 3 Clinical Suspicion • Diagnosis of WNV infection is based on a high index of clinical suspicion and obtaining specific laboratory tests. • WNV should be strongly considered in adults >50 years who develop unexplained encephalitis or meningitis in summer or early autumn within 14 days of returning from areas where there is known WNV activity (but in particular US or Canada). • It is important to bear in mind that severe neurological disease due to WNV infection has occurred in patients of all ages. It is however, much less likely in children. People over the age of 50 are about 10 times more likely than children and young people to develop severe symptoms; the risk for those over 80 years of age is almost 50-fold higher. • Year-round transmission is possible in some parts of the US. Therefore, WNV should be considered in all persons returning from the US with unexplained encephalitis and meningitis. • Any suspected cases should be reported to the Director of Public Health, as cases of suspected and confirmed viral meningitis and viral encephalitis are notifiable by clinicians and laboratory directors under the Infectious Disease (Amendment) (No. 3) Regulations 2003 (S.I. No. 707 of 2003). • EU case definitions for WNF infection are available in Appendix A on page 6. Diagnostic Testing • WNV testing is available through the National Virus Reference Laboratory. • The most efficient diagnostic method is detection of IgM antibody to WNV in serum collected within 8-14 days of illness onset or cerebrospinal fluid (CSF) collected within 8 days of illness onset using the IgM antibody capture enzyme-linked immunosorbent assay (MAC-ELISA). • Since IgM antibody does not cross the blood-brain barrier, IgM antibody in CSF strongly suggests central nervous system infection. • Patients who have been recently vaccinated against or recently infected with related flaviviruses (e.g., yellow fever, Japanese encephalitis, dengue) may have positive WNV MAC-ELISA results. Laboratory Findings Among patients in recent outbreaks in the US: • Total leukocyte counts in peripheral blood were mostly normal or elevated, with lymphocytopoenia and anaemia also occurring. • Hyponatraemia was sometimes present, particularly among patients with encephalitis. Health Protection Surveillance Centre WNV - Information for Clinicians – Aug 2005 4 • Examination of the cerebrospinal fluid (CSF) showed pleocytosis, usually with a predominance of lymphocytes. • CSF protein was universally elevated. • CSF glucose was normal. • CT scans of the brain mostly did not show evidence of acute disease, but in about 1/3 of patients, MRI showed enhancement of the leptomeninges, the periventricular areas, or both. Treatment • West Nile Fever is generally a self limiting illness, requiring simple measures only. • Ina a small proportion of cases, severe infection CNS infection may develop. In such cases, treatment is supportive, often requiring hospitalisation, intravenous fluids, respiratory support, and prevention of secondary infections. • Ribavirin in high doses and interferon α2b were found to have some activity against WNV in vitro, but no controlled studies have been completed on the use of these or other treatment options, including steroids, anti-epileptic agents or osmotic agents, in the management of WNV encephalitis. • Preclinical trials on candidate vaccines are currently underway. Further information Further information can be found at: • CDC Clinical Guidance: http://www.cdc.gov/ncidod/dvbid/westnile/clinical_guidance.htm • HPA’s website at http://www.hpa.org.uk/infections/topics_az/west_nile/menu.htm • A definitive paper, Petersen LR, Marfin AA. West Nile virus: a primer for the physician. Ann Intern Med, 2002; 137(3):173-9, gives a broad overview of WNV and its clinical management and can be accessed at http://www.annals.org/cgi/content/full/137/3/173 • A more up-to-date article Hayes EB, Komar N, Nasci RS, Montgomery SP, O'Leary DR, Campbell GL. Epidemiology and transmission dynamics of West Nile virus disease. Emerg Infect Dis [serial on the Internet]. 2005 Aug is available from http://www.cdc.gov/ncidod/EID/vol11no08/05-0289a.htm • A factsheet on West Nile Virus can be found on the Health Protection Surveillance Centre’s website at http://www.hpsc.ie/DiseaseTopicsA-Z/WestNileVirusWNV/ Dr Paul McKeown Gastroenteric/Vectorborne Unit Health Protection Surveillance Centre Health Protection Surveillance Centre WNV - Information for Clinicians – Aug 2005 August 2005 5 APPENDIX A Case Definitions for West Nile Infections The following is the European Union case definition for WNV infection and is a guide to undertaking testing for WNV: A. WNV NEUROLOGICAL SYNDROME (see notes page 8) Indications for considering the diagnosis of WNV infection and requesting a WNV test 1. Fever over 38ºC AND 2. Encephalitis or meningoencephalitis, or aseptic meningitis, or acute flaccid paralysis (poliomyelitis-like syndrome or Guillain-Barré-like syndrome) AND 3. No alternative microbiological cause identified AND 4. Occurring following exposure during a period when arboviral transmission is possible OR 5. Occurring following exposure in an area where arboviral transmission is possible OR 6. History of exposure to an alternative mode of transmission (for example through blood transfusion/organ donation). Probable Case A case presenting with indications 1, 2, 3 and 4 or 5 or 6 (as defined above) that has: 1. A positive WNV IgM test on a single serum specimen in absence of a potential alternative cause Confirmed Case A case that has: 1. Isolation of WNV from serum or CSF OR 2. Detection of WNV IgM in the CSF OR 3. Detection of WNV genomic RNA sequences in serum or CSF by RT-PCR§ OR 4. Detection of neutralising antibodies with significant titre in serum or CSF OR 5. A fourfold rise or seroconversion in WNV antibody titre in paired serum samples. § It is recognised that isolation of virus or detection of nucleic acid through PCR is unusual and the most likely confirmatory testing is through detection of neutralising antibodies or a rising antibody titre. Health Protection Surveillance Centre WNV - Information for Clinicians – Aug 2005 6 B. WNV FEVER Indications for considering the diagnosis of WNV infection and requesting a WNV test 1. Fever over 38ºC AND 2. At least one of the following: • Myalgia or • Arthralgia or • Headache or • Fatigue or • Photophobia or • Lymphadenopathy or • Maculopapular rash AND 3. Occurring during a period when arboviral transmission is possible OR 4. Occurring in an area where arboviral transmission is possible OR 5. History of exposure to an alternative mode of transmission (for example through blood transfusion/organ donation). Probable Case A case presenting with indications 1, 2 and 3 or 4 or 5 (as defined above) that has: 1. A positive WNV IgM test on a single serum specimen in absence of a potential alternative cause. Confirmed Case A case that has: 1. Isolation of WNV from serum OR 2. Detection of WNV IgM in the CSF OR 3. Detection of WNV genomic RNA sequences in serum by RT-PCR§ OR 4. Detection of neutralising antibodies with a significant titre in serum OR 5. A fourfold rise or seroconversion in WNV antibody titre in paired serum samples. § It is recognised that isolation of virus or detection of nucleic acid through PCR is unusual and the most likely confirmatory testing is through detection of neutralising antibodies or a rising antibody titre. Health Protection Surveillance Centre WNV - Information for Clinicians – Aug 2005 7 C. NOTES ON WNV NEUROLOGICAL SYNDROME Encephalitis • Altered mental state (altered level of consciousness, agitation, lethargy) and/or other evidence of cortical involvement (e.g., focal neurological findings, seizures) and • Cerebrospinal fluid (CSF) pleocytosis with predominant lymphocytes and/or elevated protein and no alternative microbiological cause identified. Meningitis • Headache, stiff neck and/or other meningeal signs and • CSF pleocytosis with predominant lymphocytes and/or elevated protein and no alternative microbiological cause identified. Acute flaccid paralysis (most cases are polio-like) • Asymmetric limb weakness without sensory loss with diminished deep tendon reflexes • Anterior horn cell disease • May have facial nerve palsy • No alternative microbiological cause identified. Health Protection Surveillance Centre WNV - Information for Clinicians – Aug 2005 8