* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download West Nile Virus - Providers - Select Health of South Carolina
Herpes simplex research wikipedia , lookup
Eradication of infectious diseases wikipedia , lookup
HIV and pregnancy wikipedia , lookup
Compartmental models in epidemiology wikipedia , lookup
2015–16 Zika virus epidemic wikipedia , lookup
Focal infection theory wikipedia , lookup
Public health genomics wikipedia , lookup
Canine distemper wikipedia , lookup
Canine parvovirus wikipedia , lookup
Multiple sclerosis research wikipedia , lookup
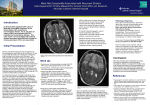
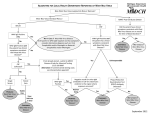
Clinical Policy Title: West Nile virus Clinical Policy Number: 17.01.07 Effective Date: Initial Review Date: Most Recent Review Date: Next Review Date: October 1, 2016 July 20, 2016 July 20, 2016 July 2017 Related policies: CP# 17.01.04 CP# 12.01.02 Policy contains: West Nile virus Immunoglobulin M antibody testing. Reverse transcriptase-polymerase chain reaction testing. Plaque-reduction neutralization testing. Zika virus Prenatal obstetrical ultrasound ABOUT THIS POLICY: Select Health of South Carolina has developed clinical policies to assist with making coverage determinations. Select Health of South Carolina’s clinical policies are based on guidelines from established industry sources, such as the Centers for Medicare & Medicaid Services (CMS), state regulatory agencies, the American Medical Association (AMA), medical specialty professional societies, and peer-reviewed professional literature. These clinical policies along with other sources, such as plan benefits and state and federal laws and regulatory requirements, including any state- or plan-specific definition of “medically necessary,” and the specific facts of the particular situation are considered by Select Health of South Carolina when making coverage determinations. In the event of conflict between this clinical policy and plan benefits and/or state or federal laws and/or regulatory requirements, the plan benefits and/or state and federal laws and/or regulatory requirements shall control. Select Health of South Carolina’s clinical policies are for informational purposes only and not intended as medical advice or to direct treatment. Physicians and other health care providers are solely responsible for the treatment decisions for their patients. Select Health of South Carolina’s clinical policies are reflective of evidence-based medicine at the time of review. As medical science evolves, Select Health of South Carolina will update its clinical policies as necessary. Select Health of South Carolina’s clinical policies are not guarantees of payment. Coverage policy Care management of persons with West Nile virus (WNV) exposure or illness is evolving. In instances where Select Health of South Carolina policies and Centers for Disease Control and Prevention (CDC) guidelines conflict, CDC guidance will govern. A. Select Health of South Carolina considers the following over-the-counter (OTC) services to be medically necessary up to plan limit in at-risk areas: Medications to relieve symptoms such as pain and fever. Environmental Protection Agency (EPA)-registered insect repellents when used as directed. EPA-registered insect repellents contain one of the following active ingredients: DEET, picaridin, IR3535, oil of lemon eucalyptus, or para-menthanediol. They are proven safe and effective when used as directed, even for pregnant and breastfeeding women. See searchable database of EPA-registered insect repellents: https://www.epa.gov/insectrepellents/find-insect-repellent-right-you. See other CDC prevention guidelines: http://www.cdc.gov/westnile/prevention/index.html. 1 B. Select Health of South Carolina considers the use of WNV testing to be clinically proven and, therefore, medically necessary when performed in accordance with CDC guidelines: Indications for WNV testing: - Symptomatic individuals as part of the differential diagnosis of febrile or acute neurologic illnesses associated with recent WNV exposure to mosquitoes, blood transfusion or organ transplantation. - Neonates whose mothers were infected with WNV during pregnancy or while breastfeeding. Test selection should consider the range of pathogens in the differential diagnosis, the criteria for classifying a WNV case as confirmed or probable, and the capability of the primary and confirming diagnostic laboratories: - Antibody testing: WNV immunoglobulin M (IgM) or immunoglobulin G (IgG) of serum or cerebrospinal fluid (CSF) within eight days of illness onset (WNV IgM preferred) using Food and Drug Administration (FDA)-approved commercial test kits or CDC-defined IgM and IgG enzyme-linked immunosorbent assay (ELISA) testing. Convalescent phase serum testing to confirm negative screen or retrospective diagnosis of infection with a specific agent. Plaque reduction neutralization test (PRNT) to confirm positive screen. - Virus isolation of CSF, serum or tissue. - WNV ribonucleic acid (RNA) testing (e.g., reverse transcriptase-polymerase chain reaction [RT-PCR]) to confirm diagnosis in acute-phase serum, CSF or tissue specimens, and to screen transplant donors in at-risk areas. Required documentation includes: 1) symptom onset date (when known); 2) date of sample collection; 3) unusual immunological status of patient (e.g., immunosuppression); 4) state and county of residence; 5) travel history (especially in flavivirus-endemic areas); 6) history of prior vaccination (e.g., yellow fever, Japanese encephalitis, or Tick-borne encephalitis viruses); and 7) brief clinical summary including clinical diagnosis (e.g., encephalitis, aseptic meningitis). C. Select Health of South Carolina considers the following services to be clinically proven and, therefore, medically necessary when performed in accordance with CDC WNV guidelines: Prenatal obstetrical ultrasound of the fetus with no upper limit on the number of tests, no sooner than two to four weeks after onset of WNV illness in the mother, unless earlier examination is otherwise indicated. For infants born to a mother with known or suspected WNV infection during pregnancy, IgM and IgG antibody serum testing and neurological and hearing examinations (See Appendix Box 1). For infants born with suspected or clinical/laboratory evidence of WNV infection, brain imaging, ophthalmalogical (including retina) evaluation, WMV IgM antibody testing of CSF, hearing screen, IgM/IgG antibody testing at six months and further examination of abnormalities as needed (See Appendix Box 2). 2 Histopathologic evaluation of the placenta and umbilical cord (See Appendix Boxes 1 and 2). Limitations: The following services are not medically necessary: - Vaccines to prevent WNV infection, as their clinical benefit has not been established. - Screening asymptomatic individuals for WNV due to the high portion of infected individuals who are asymptomatic with no associated health problems. - Treatment for WNV illness using antiviral agents, nucleic acid analogues, missense sequences, immunomodulating agents and angiotensin-receptor blockers, as their clinical benefit has not been established. For Medicare members only: Select Health of South Carolina considers the use of intravenous immunoglobulin to be medically necessary when used in the treatment of WNV infection, including meningitis and encephalitis. Alternative covered services: Standard of care for clinical examination and differential diagnosis (e.g, testing for flavivirus infection, brain imaging and ophthalmic and neurological examination). Supportive in-hospital care. Physical therapy and occupational therapy. Background WNV emerged in North America in 1999 and is most commonly spread by infected Culex mosquitoes (Centers for Disease Control and Prevention [CDC] 2015a). In a very small number of cases, WNV has spread through blood transfusions, organ transplants and from mother to baby during pregnancy, delivery or breastfeeding. As an arbovirus, WNV is a nationally notifiable condition. Arboviruses occur sporadically, and the epidemiology varies by virus and geographic area. WNV has been detected in all lower 48 states. WNV has followed a pattern of low disease intensity with periodic major epidemics with incidence peaking in 2002-2003 and again in 2012. In 2015 2,060 cases of WNV were reported to CDC, of which 1,360 cases (66 percent) were classified as neuroinvasive disease (CDC 2015a). An incubation period of 3–14 days precedes symptoms, but most people (70-80 percent) who become infected with WNV remain asymptomatic. The rest may present with abrupt onset of common nonspecific symptoms such as fever, headache, altered mental status, vomiting, diarrhea and maculopapillary rash (DeBiasi 2006). Less than one percent of people who are infected will develop a serious neurologic illness, and about 10 percent of those will die of the infection (CDC 2015a). 3 Clinical syndromes associated with WNV include West Nile fever (WNF), West Nile neuroinvasive disease (encephalitis, meningitis, poliomyelitis and other forms of acute flaccid paralysis) and ocular manifestations (e.g., chorioretinitis and vitritis); case reports of numerous other clinical manifestations have been described in association with WNV infection (Sejvar 2014). Most children with symptomatic WNV infection present with WNF; of those who develop neuroinvasive disease, meningitis is the most frequent manifestation. West Nile encephalitis is more typically manifested in older or immunocompromised individuals. Severity of neurologic illness at initial presentation does not necessarily correlate with eventual outcome. While most recover completely, recovery from severe disease may take several weeks or months, and some of the neurologic effects may be permanent (CDC 2015a). Limited data indicate muscle weakness, memory loss and difficulties with activities of daily living were among the most common long-term physical, cognitive and functional sequelae, respectively (Patel 2015). Key risk factors for developing neuroinvasive disease include increasing age, underlying morbidity (cardiovascular disease or cancer), immune status and specific genetic factors often linked with innate and acquired immunity (Tyler 2014, Patel 2015). Detection and diagnosis: Diagnosis is based on clinical symptoms, laboratory testing, recent exposure and vaccination history. Laboratory diagnosis includes detection of viable WNV, WNV RNA and WNV-specific antibodies. Laboratory diagnosis is generally accomplished by testing for WNV-specific IgM antibodies in serum or CSF, WNV IgG antibody testing, PRNTs and virus cultures of CSF. Nucleic acid amplification tests (NAATs) (e.g., RT-PCR) in serum, CSF and tissue specimens amplify and measure genetic material to detect the presence of WNV RNA. NAATs are routinely used to screen units of donated blood for WNV and may be performed on the blood of tissue and organ donors prior to transplantation. NAATs and viral cultures are used frequently in research settings. Immunoassays for WNV-specific IgM are available commercially and through state public health laboratories. FDA has approved four commercially-available test kits from different manufacturers for detection of WNV IgM and IgG antibodies, and CDC-defined IgM and IgG enzyme-linked immunosorbent assay (ELISA) may be used (Table 1) (CDC 2013a, FDA 2016). Table 1. Characteristic sensitivity and time required for WNV virus or viral RNA human detection assays1 Test Virus isolation in suckling mouse Virus isolation in Vero cell culture Detects Infectious virus Infectious virus Detection level (plaque forming units [pfu]/ml) 100 100 Assay time 4-10 days 3 days 4 Standard RT-PCR Nucleic Acid Sequence-based Amplification (NASBA) TaqMan® RT-PCR Transcription Mediated Amplification 1 Adapted from CDC (2013). Viral RNA 5 8 hours Viral RNA 0.1 4 hours Viral RNA Viral RNA 0.1 0.02 4 hours 4 hours Searches Select Health of South Carolina searched PubMed and the databases of: UK National Health Services Centre for Reviews and Dissemination. Agency for Healthcare Research and Quality’s National Guideline Clearinghouse and other evidence-based practice centers. The Centers for Medicare & Medicaid Services (CMS). We conducted searches on June 13, 2016. Search terms were: "West Nile virus"[Mesh]), "West Nile Virus vaccines"[Mesh], “West Nile fever” and the free text term “West Nile virus.” We included: Systematic reviews, which pool results from multiple studies to achieve larger sample sizes and greater precision of effect estimation than in smaller primary studies. Systematic reviews use predetermined transparent methods to minimize bias, effectively treating the review as a scientific endeavor, and are thus rated highest in evidence-grading hierarchies. Guidelines based on systematic reviews. Economic analyses, such as cost-effectiveness, and benefit or utility studies (but not simple cost studies), reporting both costs and outcomes — sometimes referred to as efficiency studies — which also rank near the top of evidence hierarchies. Findings One literature review by CDC (2015b) and six evidence-based guidelines from CDC (2004, 2013b and 2015c), the Infectious Disease Society of America (IDSA) (Tunkel, 2008), the American College of Radiologists (ACR) (ACR 2014) and the American Society for Reproductive Medicine/Society for Assisted Reproductive Technology (ASRMS) (2008) provide the basis for this policy. WNV disease should be considered in the differential diagnosis of febrile or acute neurologic illnesses associated with recent exposure to mosquitoes, blood transfusion or organ transplantation, and of illnesses in neonates whose mothers were infected with WNV during pregnancy or while breastfeeding. Numerous pathogens cause encephalitis, aseptic meningitis and febrile disease with clinical presentations similar to those caused by WNV and should be considered in the differential diagnosis. Testing should be performed in symptomatic individuals, not for screening asymptomatic individuals due to the high proportion of infected individuals who are asymptomatic with no associated health problems. Selection of diagnostic test procedures should take into consideration the range of pathogens 5 in the differential diagnosis, the criteria for classifying a WNV case as confirmed or probable, as well as the capability of the primary and confirming diagnostic laboratories. WNV IgM antibody testing in serum or CSF is preferred to initially screen for WNV infections. The sensitivity of PCR testing is inferior to IgM testing, as peak viremia occurs three to four days before symptom onset. WNV-specific IgM antibodies are usually detectable in the acute phase three to eight days after onset of symptoms and persist for 30 to 90 days and possibly longer. The presence of virusspecific IgM in CSF is usually indicative of neuroinvasive disease, because IgM antibodies do not readily diffuse across the blood-brain barrier. If serum is collected within eight days of illness onset, the absence of detectable virus-specific IgM does not rule out infection, and the test may need to be repeated on a later sample. In general, IgG antibodies are detectable shortly after the appearance of IgM antibodies and persist for years following a symptomatic or asymptomatic infection. The presence of IgG antibodies alone is only evidence of previous infection. Presence of IgM or IgG antibodies may reflect cross-reactivity with other flaviviruses or non-specific reactivity. Clinically compatible cases with the presence of IgG, but not IgM, should be evaluated for other etiologic agents. PRNT should be used to confirm all positive antibody tests to differentiate flavivirus infections. At the time of initial presentation, serum samples should be stored and tested at a later time with convalescent phase serum samples. Such testing is indicated for the retrospective diagnosis of infection with a specific agent rather than for initiating therapy. A fourfold or greater change in WNV-specific neutralizing antibody titer between acute- and convalescent-phase serum samples collected two to three weeks apart confirms acute infection. Virus culture and RT-PCR testing to detect WNV RNA in serum or CSF of clinically ill, immunocompetent patients have limited utility in diagnosing human WNV neuroinvasive disease due to the low level viremia present in most cases at the time of clinical presentation. However, these tests may prove useful in immunocompromised patients, in whom antibody development is delayed or absent. Negative virus culture and RT-PCR results should not be used to rule out an infection. Routine clinical laboratory studies are generally nonspecific. In patients with neuroinvasive disease, typical CSF findings, particularly in West Nile meningitis, include pleocytosis (generally less than 500 cells/mm3) with elevated protein but normal glucose levels. Features of the pleocytosis that are indicative of WNV infection include a prolonged predominance of polymorphonuclear cells and the presence of abnormal-appearing reactive lymphocytes or monocytes, including plasma cell-like and mollaret-like cells. 6 Computed tomography (CT) and MRI are most frequently used to evaluate patients with possible CNS infection, with MRI being preferred when available. The incidence of acute MRI abnormalities in patients with WNV neuroinvasive disease has been extremely variable, but MRI may detect early changes in the basal ganglia, thalamus, and brainstem in approximately 30 percent of patients with encephalitis, and in the anterior spinal cord in patients with poliomyelitis. Diffusion-weighted MRI is superior to conventional MRI and is the preferred neuroimaging modality. CT with and without intravenous contrast administration should be used only if MRI is unavailable, impractical or cannot be performed. Prevention and treatment: There are no effective vaccines to prevent WNV infection. Several have completed phase I or phase II human clinical trials with promising results, but lack of predictability of the magnitude and location of outbreaks are problematic for designing phase III trials. Prevention includes avoiding mosquito bites (e.g., mosquito repellant and clothing) and reducing the number of mosquitos (e.g., eliminating standing water). There is no effective treatment for WNV infection. Milder cases are generally self-limiting or can be treated with over-the-counter medications to relieve symptoms such as pain and fever. In more severe cases, patients often need to be hospitalized to receive supportive treatment and rehabilitation to address neurological and cognitive consequences of CNS infection. There is empiric use without proof of benefit of several therapeutic modalities, including antiviral agents, nucleic acid analogues, missense sequences, immunomodulating agents and angiotensin-receptor blockers. Perinatal and reproductive considerations: Pregnant women are not at higher risk for WNV infection. Neither the proportion of WNV infections during pregnancy that result in congenital infection nor the spectrum of clinical abnormalities associated with congenital WNV infection is known. The risk of transmitting WNV through breastfeeding to the newborn appears to be very low. In light of the well-established health benefits of breastfeeding, there are no recommendations for a woman to stop breastfeeding because of WNV illness. Pregnant women and women who are breastfeeding should take preventive measures, including insect repellant, to reduce their risk for WNV infection (CDC 2015). CDC recommends clinical evaluation of infants born to mother with known or suspected WNV infection during pregnancy (See Appendix). Practitioners should delay donation of gametes from donors who have confirmed or suspected WNV infections until 14 days after the condition is resolved or 28 days from the onset of symptoms, whichever is later. Donors who are not in good health, including those with recent significant fever and flu-like illnesses or recent viral meningitis, encephalitis or meningoencephalitis episodes, should be similarly deferred. 7 Summary of clinical evidence: Citation CDC (2015b) WNV therapeutics CDC (2015c) Pregnancy & breastfeeding CDC (2013b) Guidelines for surveillance, prevention and control Tunkel (2008) for the IDSA Guideline-management of encephalitis ACR (2014) Practice parameter for MRI Content, Methods, Recommendations Key points: No antiviral or adjunctive therapies are approved or recommended for treatment of WNV disease. Case reports and case series of various products (e.g., standard and hyperimmune polyclonal immune globulin, monoclonal immune globulin, interferon, ribavirin and corticosteroids) were inconclusive; controlled clinical trials have shown no benefit for infections due to WNV or closely related flavivirus infections. Key points: Pregnant women are not at higher risk for WNV infection. Risk of transmission from infected pregnant woman to fetus or newborn is low. Risk for WNV transmission through breastfeeding is unknown. Breastfeeding should be continued. Preventive measures, including insect repellant, should be used. Key points: No effective vaccine. Testing recommended for symptomatic individuals only. WNV IgM antibody assay is preferred initial screening test. o Confirm positive results using CDC-defined IgM and IgG ELISA and PRNT on specimens against other flaviviruses known to be active or be present in the area or in the region where the patient traveled. Virus isolation of CSF or serum and RT-PCR of serum or CSF to detect WNV RNA in clinically ill patients have limited utility in diagnosing human WNV neuroinvasive disease due to low level viremia present in most cases at clinical presentation. May be helpful in confirming human WNV infection in immunocompromised patients when antibody development is delayed or absent. Key points: Serum IgM recommended. Recommend storing serum for testing later with convalescent phase serum samples for retrospective diagnosis of infection with a specific agent. A 4-fold increase in convalescent-phase IgG antibody titers may be necessary to establish the diagnosis. CSF IgM recommended over CSF PCR due to superior sensitivity. PRNT recommended in areas where multiple flaviviridae co-circulate or in patients who have received previous vaccination against a related arbovirus. CSF virus culture not routinely recommended. NAATs preferred. Neuroimaging recommended for evaluating encephalitis. MRI superior to CT. T2weighted and fluid-attenuated inversion recovery (FLAIR) MRI findings (∼30% of cases) preferred over standard MRI. May reveal hyperintense lesions in the substantia nigra, basal ganglia, and thalami; similar lesions in the spinal cord. o Use CT only if MRI unavailable. Supportive care only recommended. Ribavirin not recommended. Key points: MRI recommended for infectious disorders: encephalitis, meningitis, abscess. Most commonly accepted protocols are T1-weighted sequence in the sagittal plane (or a T1-weighted volumetric acquisition) and T2-weighted fluid-attenuated inversion recovery 8 Citation ASRMS (2008) Position statement for gamete donation CDC (2004) Infants with congenital exposure (see Appendix) Content, Methods, Recommendations (FLAIR) and fast spin-echo or turbo-spin-echo (or equivalent) sequences in the axial plane. o If FLAIR is not available or in children under the age of 2 years, proton density weighted sequences may be helpful. Under certain clinical circumstances, very rapid acquisitions such as echo planar imaging or single shot fast-spin-echo imaging can be performed to obtain T2 information. Key points: No definitive evidence linking WNV transmission with reproductive cells. Recommend practitioners defer gamete donors who have confirmed or suspected WNV infections (until 14 days after the condition is resolved or 28 days from the onset of symptoms, whichever is later). Donors who are not in good health, including those with recent significant fever and flulike illnesses or recent viral meningitis, encephalitis or meningoencephalitis episodes, should be similarly deferred. Key points: Screening asymptomatic pregnant women for WNV infection not recommended. Recommend serum (and CSF, if clinically indicated) WNV antibody testing in pregnant women who have meningitis, encephalitis, acute flaccid paralysis, or unexplained fever in at-risk area: o If testing is positive, report to local or state health department, and women followed to determine pregnancy outcome. Consider detailed prenatal obstetrical ultrasound examination to evaluate for structural abnormalities no sooner than two to four weeks after onset of illness in the mother, unless earlier examination is otherwise indicated. Amniotic fluid, chorionic villi or fetal serum can be tested, but sensitivity, specificity, and predictive value are unknown, and the clinical consequences of fetal infection have not been determined. In cases of miscarriage or induced abortion, advise testing of all products of conception (e.g., the placenta and umbilical cord) for evidence of infection to document effects of WNV infection on pregnancy outcome. Recommend clinical evaluation of infant born to mother with known or suspected WNV infection during pregnancy. Consider further evaluation if any clinical abnormality found or laboratory testing indicates possible congenital WNV infection. Glossary Acute flaccid paralysis — A heterogeneous neurologic condition affecting the anterior horn of the spinal cord. Defined by the onset of acute, areflexic or hyporeflexic weakness in one or more limbs and/or the cranial nerve-innervated muscles (e.g., seen in poliomyelitis and Guillain-Barré syndrome). Arbovirus — A class of viruses transmitted to humans by arthropods such as mosquitoes and ticks. Pleocytosis — Elevated inflammatory cells in CSF (> 5 leukocytes/mm3). May occur with peripheral leukocyte count >10,000/mm3 or neuroimaging findings consistent with acute meningeal inflammation. 9 West Nile encephalitis — WNV infection of the brain parenchyma itself. More typically manifested in older or immunocompromised individuals. West Nile fever — A symptom that occurs typically between two and 15 days after WNV infection. May occur in isolation or with other symptoms of WNV infection. West Nile meningitis — WNV infection of the meninges (the outer covering of the brain and spinal cord). Comprises the largest percentage of neuroinvasive disease cases in younger age groups. West Nile poliomyelitis — A form of acute flaccid paralysis resulting from WNV infection of the anterior horn cells of the spinal cord, leading to acute flaccid limb weakness. West Nile virus (WNV) — A neurotropic species of flavivirus transmitted to humans mainly by Culex mosquitoes. Also transmitted by blood transfusions, organ transplantation or from mother to fetus or baby during pregnancy, delivery or breastfeeding. References Professional society guidelines/other: American Society for Reproductive Medicine/Society for Assisted Reproductive Technology position statement on West Nile virus. Fertil Steril. 2008; 90(5 Suppl): S270 – 271. ACR–ASNR–SPR Practice Parameter for the Performance and Interpretation of Magnetic Resonance Imaging (MRI) of the Brain. ACR website. http://www.acr.org/~/media/ACR/Documents/PGTS/guidelines/MRI_Brain.pdf. Accessed June 17, 2016. CDC. Interim guidelines for the evaluation of infants born to mothers infected with West Nile virus during pregnancy. MMWR Morb Mortal Wkly Rep. 2004; 53(7): 154 – 157. CDC. Pregnancy & Breastfeeding. Last updated March 31, 2015. CDC website. http://www.cdc.gov/westnile/faq/pregnancy.html. Accessed June 20, 2016.(c) CDC. West Nile virus disease therapeutics. Review of the literature for healthcare providers. Revised June 15, 2015. CDC National Center for Emerging and Zoonotic Infectious Diseases website. http://www.cdc.gov/westnile/resources/pdfs/wnv-therapeutics-summary.pdf. Accessed June 14, 2016.(b) CDC. West Nile Virus in the United States: Guidelines for Surveillance, Prevention, and Control. 4th revision. June 14, 2013. CDC website. http://www.cdc.gov/westnile/resources/pdfs/wnvguidelines.pdf. Accessed June 13, 2016.(b) 10 Tunkel AR, Glaser CA, Bloch KC, et al. The management of encephalitis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2008; 47(3): 303 – 327. Peer-reviewed references: 510(k) Premarket Notification using product code NOP. FDA website. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm. Accessed June 17, 2016. Beasley DW. Vaccines and immunotherapeutics for the prevention and treatment of infections with West Nile virus. Immunotherapy. 2011; 3(2): 269 – 285. CDC.General Questions About West Nile Virus. Last updated March 30, 2015. CDC website. http://www.cdc.gov/westnile/faq/genquestions.html. Accessed June 13, 2016.(a) CDC. West Nile virus and other arboviral diseases--United States, 2012. MMWR Morb Mortal Wkly Rep. 2013; 62(25): 513 – 517.(a) Debiasi RL, Tyler KL. West Nile virus meningoencephalitis. Nat Clin Pract Neurol. 2006; 2(5): 264 – 275. Patel H, Sander B, Nelder MP. Long-term sequelae of West Nile virus-related illness: a systematic review. Lancet Infect Dis. 2015; 15(8): 951 – 959. Sejvar JJ. Clinical manifestations and outcomes of West Nile virus infection. Viruses. 2014; 6(2): 606 – 623. Tyler KL. Current developments in understanding of West Nile virus central nervous system disease. Curr Opin Neurol. 2014; 27(3): 342 – 348. Clinical trials: Searched clinicaltrials.gov on June 20, 2016 using terms West Nile virus | all studies. 19 studies found, two still active but not recruiting. Evaluating the Safety and Immunogenicity of a Live Attenuated West Nile Virus Vaccine for West Nile Encephalitis in Adults 50 to 65 Years of Age. ClinicalTrials.gov website. https://ClinicalTrials.gov/show/NCT02186626. Published July 8, 2014. Updated December 14, 2015. Accessed June 20, 2016. Phase 1 Trial of Inactivated West Nile Virus Vaccine. ClinicalTrials.gov website. https://ClinicalTrials.gov/show/NCT02337868. Published January 8, 2015. Updated June 16, 2016. Accessed June 20, 2016. 11 CMS National Coverage Determinations (NCDs): No NCDs identified as of the writing of this policy. Local Coverage Determinations (LCDs): L34175 Ophthalmic Angiography (Fluorescein and Indocyanine Green). CMS website. http://www.cms.gov/medicare-coverage-database/details/lcd-details.aspx?LCDId=34175&ver=10. Accessed June 20, 2016. L34426 Ophthalmic Angiography (Fluorescein and Indocyanine Green). CMS website. http://www.cms.gov/medicare-coverage-database/details/lcd-details.aspx?LCDId=34426&ver=9. Accessed June 20, 2016. A52446 Intravenous Immune Globulin (IVIG) - Related to LCD L33394. CMS website. http://www.cms.gov/medicare-coverage-database/details/article-details.aspx?articleId=52446&ver=16. Accessed June 20, 2016. IVIG is covered when used in the treatment of WNV infection, including meningitis and encephalitis. Commonly submitted codes Below are the most commonly submitted codes for the service(s)/item(s) subject to this policy. This is not an exhaustive list of codes. Providers are expected to consult the appropriate coding manuals and bill accordingly. CPT Code 86788 86789 Description West Nile virus, IgM West Nile virus Comments ICD-10 Code A92.3039 Z20.828 Description Comments HCPCS Code Description West Nile virus Exposure to communicable viral disease Comments Appendix. 12 CDC interim guidelines for the evaluation of WNV infection in infants born to mothers infected with WNV during pregnancy (2004)1 13 14 1 Adapted from CDC (2004). 15