* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download West Nile Virus
Marburg virus disease wikipedia , lookup
Ebola virus disease wikipedia , lookup
Sexually transmitted infection wikipedia , lookup
Chagas disease wikipedia , lookup
Middle East respiratory syndrome wikipedia , lookup
Henipavirus wikipedia , lookup
Hepatitis B wikipedia , lookup
Epidemiology of HIV/AIDS wikipedia , lookup
Microbicides for sexually transmitted diseases wikipedia , lookup
Diagnosis of HIV/AIDS wikipedia , lookup
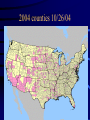
Black Bugs Blood: West Nile Virus & the Blood Supply Infectious Transfusion Risks, Screening Blood donations for WNV and other icky things Jed Gorlin, Memorial Blood Centers Duluth TAM 11/04 Risks of Transmission • Infectious Risks – – – – – Viral Bacterial Protozoa Ricketsia Other • ?Prion • Non-infectious risks – – – – – – Transfusion Reaction Metabolic Cardiac Overload Dilutional Coagulopathy TAGVHD Alloimmunization Transfusion Safety • Product Safety – Donor Recruitment – Donor history screening – Donor Testing – Manufacturing cGMP • Transfusion Safety – – – – – – – Patient blood sample Med indication for Tx. Special Tx needs Select right unit Issue to floor administration monitoring & evaluation of reaction 108 Paling Risk 6Scale for Major4 Transfusion Hazards 7 5 3 10 10 10 10 10 102 101 HIV HCV 100 General anesthesia HBV Bacteria Mis-Transfusion TRALI TA-GVHD Cardiac Metabolic risk in neonates Sunny Dzik, MD Under transfusion Window period risk • Why is there any residual risk? There is potential for transfusion transmission, if donors is drawn after acquiring the disease but before they make an antibody response. • Time from infectivity to test reactivity • Chance of transmission is a function of both incidence and length of window period. NAT screening (HCV/HIV/HBV) • Two major testing platforms for HIV/HCV • Roche-Pools of 24, separate tests for HCV, HIV. Advantage: automated detection, disadvantage, very manual sample prep • Chiron-Gen Probe (TMA) Multiplex test – Pools of 16, automated sample prep, manual detection. Requires extra round to resolve positive samples. More false positives MBC Experience • After testing almost 2,000,000 samples, MBC has detected 1 HIV NAT window period case and 5 HCV NAT+/EIA negative samples. • Almost 1,000,000 samples were tested for both HCV and HIV before a single NAT+/EIA - sample was found. U.S. NAT Program Yields Program # HCV Rate # HIV Rate UBS 16 1:294,926 2 1:2,359,409 ABC-Roche 23 1:239,630 1 1:2,581,512 AIBC 2 1:236,937 0 0:473,874 ARC 29 1:331,828 1 1:9,623,012 US Total 70 1:275,830 4 1:3,759,451 What Has NAT Testing Cost the US?? • Assume 13 million blood donations annually • Assume an average cost/donation of $16 • Cost of HCV/HIV- NAT = • $208,000,000/yr or $104M each Cost/HCV-NAT positive donation: • Average HCV-NAT pos. rate in US = 1:276,000 donations • 13 million donations/yr collected • 47 window case donations expected @ $104,000,000 total • Cost/HCV-NAT positive donation detected = $>2,200,000/donation Cost/HIV-NAT positive donation: • Average HIV-NAT pos. rate in US = 1:3,760,000 donations • 13 million donations/yr collected • 3.5 window case donations expected @ $104,000,000 total • Cost/HIV-NAT positive donation detected = > $28,000,000/donation Cost-Effectiveness Comparisons Cost-effectiveness ($/YLE) 8,000,000 6,000,000 Transfusion Safety Interventions 4,000,000 2,000,000 600,000 400,000 200,000 Commonly Accepted Medical Practices RhIg/HDN HTN Annual Cardiac CABG Prophy- Therapy Mammo- Trans(one gram plantation vessel) laxis HCV Lookback PADCABG ALT Anti-HBc MP NAT p24 Ag MP=>SD Testing Testing Testing HIV+HCV NAT for HIV SD FP Other Transfusable Parasitic • Chagas – Trypanosoma cruzi Endemic: Central & So. America – Infected reduviid (kissing) bug falls from thatched roof, defecates and inoculates skin – May be under-recognized cause of heart failure – Only 7 cases Tx transmission in US/Canada – Screened for in Brazil and other LA countries – ARC proposes to implement screening – MBC to participate in Chagas trial ~1/05 West Nile Virus: Background and Ecology West Nile Virus: Background and Ecology • First isolated in West Nile district, Uganda, 1937 • Commonly found in humans and birds and other vertebrates in Africa, Eastern Europe, West Asia, and the Middle East, but has not previously been documented in the Western Hemisphere • Basic transmission cycle involves mosquitoes feeding on birds infected with the West Nile virus • Infected mosquitoes then transmit West Nile virus to humans and animals when taking a blood meal The Japanese Encephalitis Serocomplex of the Family Flaviviridae 1999 - 2002 Verified WNV Surveillance Results Reported to ArboNet Year States Humans/ Fatalities Birds Mosquito Pools Horses 1999 4 62/6 ? 16 25 2000 11 + DC 21/2 4305 515 63 2001 27 + DC 66/9 7332 919 731 2002 44 + DC 3949/262 14,790 5938 11,450 Date of Symptom Onset, West Nile Virus United States, 1999-2001 Number of cases 30 2001 2000 1999 25 20 15 10 5 0 Week ending Clinical Epidemiology • • • • Incubation period 3 - 14 days ~80% of infections are asymptomatic 20% develop “West Nile fever” 1 in 150 develop meningoencephalitis – Advanced age primary risk factor for severe neurological disease and death Outcome of West Nile Virus Infection among Hospitalized Patients • At discharge (NY and NJ, 2000) – More than half did not return to functional level – Only one-third fully ambulatory • At one year (NYC 1999 patients) – Fatigue 67%, memory loss 50%, difficulty walking 49%, muscle weakness 44%, depression 38% Summer 2003 • • • • Implemented WNV NAT screening 7/1/03 Automated DNA extraction Pool size 6-dedicated pooling machines TaqMan platform requires lots of room. Total NAT laboratory space doubled • MBC detected ~36 WNV+ blood donors, mostly in Nebraska, South Dakota and Iowa More Automated System Hamilton Pipettor COBAS TaqMan (96/48) COBAS AmpliPrep WNV Human cases and deaths State Colorado Nebraska South Dakota Texas North Dakota Wyoming Pennsylvania Total Cases 2647 1942 1039 717 617 375 237 9862 Deaths 61 29 14 37 5 9 8 264 + WNV rate weekly (6/30-9/30) WNV Incidence 0.800% 0.700% 0.600% % Positive 0.500% CBBLAN MBC 0.400% Siouxland SFSV+Avera 0.300% 0.200% 1 / 500 0.100% 0.000% 1 2 3 4 5 6 7 Week 8 9 10 11 12 13 2004 map as of 10/26/04 2004 counties 10/26/04 WNV+ blood donors - 10/26/04 2004 WNV Transfusion transmission in Arizona • MMWR Sept 17, 2004 p 842 • In 2003 blood centers interdicted ~800 blood components via pooled testing. Because of 6 cases of transfusion transmission, a policy for single donor (SD) testing was implemented for 2004. • 3 days before switch to SD in Arizona (from TMA pool of 16), a 43 yo with severe diabetes was transfused following a knee amputation. He subsequently developed WNV and died. The units were traced and one donor was shown to be WNV+ by SD but not pooled testing. Implications • Despite reduced pool size and plans to implement single donor testing, window period cases of WNV continue to occur. • There have been two HIV transmissions despite pooled NAT testing • Both manufacturers are working to create more automated systems that facilitate single donor testing. These will be more expensive, but will allow greater throughput than current manual tests