* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 4.1 Refinements of the Atomic Model
Photon polarization wikipedia , lookup
Wave packet wikipedia , lookup
Old quantum theory wikipedia , lookup
Uncertainty principle wikipedia , lookup
Electron scattering wikipedia , lookup
Relational approach to quantum physics wikipedia , lookup
Double-slit experiment wikipedia , lookup
Introduction to quantum mechanics wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
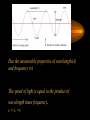
4.1 Refinements of the Atomic Model Mr. Chris Walters Williamstown Schools The Dual Nature of Light • Experimentation has shown that light behaves as both a wave (energy) and as a particle (matter). • This concept seems to defy logic but is supported. • “A form of energy that displays wavelike behavior as it travels through space” • Travels at 3.0 x 108 m/sec in a vacuum (c) Has the measurable properties of wavelength() and frequency () The speed of light is equal to the product of wavelength times frequency. c=λ •ν All the wavelengths of light form a CONTINUOUS SPECTRUM FREQUENCY is measured in HERTZ (Hz). A Hz is one wave occurring per second. Visible Light • that portion of the electromagnetic spectrum from wavelengths 375 nm to 725 nm • Illustrated in 7 colors ROY G BIV (from longest wavelength to shortest) Light as Particles • The wave theory of light encountered two problems – It predicted that heated objects would give off ultraviolet light, they emit visible light – It could not explain the Photoelectric effect Photoelectric Effect • The Photoelectric effect is the emission of electrons by metals when light shines on them – Only certain frequencies of light caused the effect Explanation - Planck • Max Planck (1858-1947) proposed that energy was given off in specific quantities or Quanta. (singular: Quantum) • Planck proposed the following equation E= h, where h was a constant value. • Planck’s constant (h) = 6.626 x 10 -34 Continuation - Einstein • An individual particle of light is called a photon. • Einstein proposed that absorption of photons at certain quanta explained the photoelectric effect. Exciting Electrons to release light • When atoms in the gaseous state are heated they increase in PE then return to original state as they emit light. – As they are heated the electrons leave the ground state and become excited. – They give off light in specific amounts (quanta). – This can be illustrated in the line spectrum Bohr Model of the Atom • Niels Bohr (1885-1962) proposed a model using this new information. • It showed the orbits of the electrons a specific quantized distances from the nucleus. • Bohr’s model worked well on Hydrogen, but failed to match data with more complicated atoms and their line spectra. Spectroscopy • A spectroscope is an instrument that separates light into a spectrum that can be analyzed. • This allows the light to be examined and its atomic source discovered. Quantum Model • Replaced the orbits of Bohr with regions of space in which the electrons are located called orbitals. • Orbitals are quantized. • Quantum theory mathematically explains the wave properties of electrons and other small particles. Louis deBroglie • Louis deBroglie (18921987) proposed the dual nature of electrons. Schrodinger’s Wave Equation • Erwin Schrödinger proposed his wave equation that mathematically explains electron action. Heisenberg’s Uncertainty Principle • Werner Heisenberg contributed his Uncertainty Principle which states it is not possible to know both the velocity and position of a particle at the same time.