* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Regulation of Cardiac Output by Stroke Volume and Heart Rate in
Coronary artery disease wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Heart failure wikipedia , lookup
Myocardial infarction wikipedia , lookup
Electrocardiography wikipedia , lookup
Cardiac surgery wikipedia , lookup
Jatene procedure wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
Heart arrhythmia wikipedia , lookup
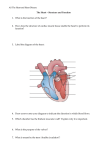
CONTROL OF CARDIAC OUTPUT/Vatner and Boettcher 30. 31. 32. 33. 34. recoiling forces of excised cat lungs. J Appl Physiol 14: 905-908, 1959 Hauge A, Bo G, Waaler BA: Interrelations between pulmonary liquid volumes and lung compliance. J Appl Physiol 38: 608-614, 1975 Lenfant C, Howell BJ: Cardiovascular adjustments in dogs during continuous pressure breathing. J Appl Physiol 14: 425-428, 1960 Staub NC, Nagano H, Pearce ML: Pulmonary edema in dogs, especially the sequence of fluid accumulation in the lungs. J Appl Physiol 22: 227-240, 1967 Howell, JBL, Permutt S, Proctor DF, Riley RL: Effects of inflation of the lung on different parts of the pulmonary vascular bed. J Appl Physiol 16: 71-76, 1961 Mead J, Takishima T, Leith D: Stress distribution in lungs: A model 557 of pulmonary elasticity. J Appl Physiol 28: 596-608, 1970 35. Guyton AC: A concept of negative interstitial pressure based on pressures in implanted perforated capsules. Circ Res 12: 399-414, 1963 36. Guyton AC, Taylor AE, Granger HJ: Circulatory Physiology. II: Dynamics and Control of Body Fluids. Philadelphia, Saunders, 1975, pp 18-26 and 166-180 37. Guyton AC, Taylor AE, Drake RE, Parker JC: Dynamics of subatmospheric pressure in the pulmonary interstitial fluid. In Lung Liquids, Ciba Foundation Symposium 38. Amsterdam, Elsevier, 1976, pp 75-95 38. Guyton, AC: Interstitial fluid pressure. II. Pressure-volume curves of interstitial space. Circ. Res. 16: 452-460, 1965 Regulation of Cardiac Output by Stroke Volume and Heart Rate in Conscious Dogs Downloaded from http://circres.ahajournals.org/ by guest on April 30, 2017 STEPHEN F. VATNER AND DEDO H. BOETTCHER SUMMARY We examined the relative importance of increases in stroke volume and heart rate in mediating increases in cardiac output in response to elevations in preload, inotropic state, or a combination of these factors in 15 conscious dogs with love, physiological heart rates. Elevating preload by volume loading with saline increased left atrial pressure by 15 mm Hg, cardiac output by 147 ± 7% from a control of 2340 ± 80 ml/min, and heart rate by 143 ± 7% from a control of 62 ± 2 beats/min, but did not alter stroke volume. Similarly, volume loading with blood increased cardiac output by 100 ± 5% and heart rate by 108 ± 10%, while stroke volume did not change significantly. Hemorrhage in conscious dogs reduced cardiac output by 49 ± 4% and stroke volume by 75 ± 2% while increasing heart rate by 113 ± 15%. In dogs anesthetized with pentobarbital Na, and with an open chest, volume loading increased stroke volume by 243 ± 89% but did not alter heart rate. In conscious dogs, isoproterenol increased cardiac output solely by increasing heart rate, failing to increase stroke volume, whereas dobutamine, a sympathomimetic amine with less positive chronotropic action than isoproterenol, raised stroke volume by approximately 25%. Infusion of both sympathomimetic amines in the volume-loaded state increased stroke volume by a slightly greater amount than either volume loading or sympathomimetic amine infusion by itself. Severe exercise also increased stroke volume by 27 ± 2%, whereas cardiac output rose by 402 ± 24%. Thus, in the conscious dog with a low physiological heart rate, stroke volume is relatively large at rest and does not increase at all, even with maximally tolerable volume loading, and only modest increases were observed with exercise or combined sympathomimetic amine infusion and volume loading. WHEREAS IT IS OBVIOUS that the cardiac pump can increase its output only through a change in frequency or a change in stroke volume, the relative importance of these two factors in mediating changes in preload remains controversial despite intense investigation of this subject during the past century. Starling's pioneering work on the heart-lung preparation established a dominant role for stroke volume in mediating changes in cardiac output.' From the Departments of Medicine, Harvard Medical School and Peter Bent Brigham Hospital, and Department of Cardiology, Childrens Hospital Medical Center, Boston, Massachusetts, and the New England Regional Primate Research Center, Southboro, Massachusetts. Supported in part by U.S. Public Health Service Grants HL 15416. 17459, HL 10436, AHA 73-833, and NSG 2136. Dr. Vatner is an Established Investigator of the American Heart Association. Dr. Boettcher was an International Research Fellow of the U.S. Public Health Service. Presented in part at the Meeting of Federation of American Societies for Experimental Biology, Atlantic City. New Jersey, April, 1975. Address for reprints: Stephen F. Vatner, M.D., New England Regional Primate Research Center, One Pine Hill Drive, Southboro, Massachusetts 01772. Original manuscript received June 10, 1976; accepted for publication December 13, 1977. Rushmer, using a normal conscious animal model,2"' challenged the views of Starling and concluded that stroke volume remained roughly constant, particularly when cardiac output rose during exercise.2 However, Rushmer and colleagues did not examine the effects on stroke volume of elevating preload by volume loading, a case in which a large rise in stroke volume might be predicted. The goal of this study was to determine the extent to which stroke volume changed in response to marked alterations in preload, starting from the basal state, i.e., a reclining conscious dog with a physiological heart rate. It was considered essential to study animals with low, physiological heart rates, since the importance of changes in stroke volume tend to be overemphasized when the baseline heart rate is elevated, as occurs in excited animals, or during general anesthesia. To examine responses to marked changes in preload, trained dogs instrumented with electromagnetic flow probes on the aorta were studied when cardiac output was elevated by increasing preload to the maximum tolerated by the conscious dogs and when cardiac output was reduced by diminishing preload. CIRCULATION RESEARCH 558 To ascertain maximal increases in stroke volume, two cardioactive sympathomimetic amines, isoproterenol, a j8-adrenergic agent with positive inotropic and chronotropic properties and dobutamine, an inotropic agent with a relatively weak positive chronotropic effect,'1 were infused in the presence of elevated preload. Finally, we examined a physiological situation, in which both preload and the inotropic state rise, i.e., maximal exercise. By studying these interventions, we obtained a comprehensive picture of the regulation of cardiac output and stroke volume under conditions of altered preload and inotropic state in the normal, conscious dog. Methods Downloaded from http://circres.ahajournals.org/ by guest on April 30, 2017 Twenty four mongrel dogs weighing 18-25 kg were anesthetized with pentobarbital, Na, 30 mg/kg, iv. Through a left thoracotomy in the 4th intercostal space, an electromagnetic flow probe (Zepeda Instruments) was implanted around the ascending aorta, and heparin-filled Tygon catheters were implanted in the aorta and the left atrium. Arterial and atrial pressures were measured with the catheters and Statham P23Db strain gauge manometers (Statham Instruments). A square wave electromagnetic flowmeter (Benton Instruments) was used to measure cardiac output and stroke volume. A modification of the Benton miniature electromagnetic flowmeter was used to measure and radiotelemeter stroke volume and cardiac output during severe exercise. Flow probes were calibrated in vitro with a gravity flow system and crosscalibrated in vivo by using the radioactive microsphere technique to measure cardiac output. The experiments were conducted 2-6 weeks postoperatively when the dogs had recovered from operation and were fully accustomed to the laboratory and personnel. Control records of cardiac output, heart rate, and arterial and atrial pressures were obtained while the unsedated dogs were lying quietly and during the various interventions. Experiments were discarded if the dogs showed signs of arousal or excitement or if baseline heart rate was VOL. 42, No. 4, APRIL 25% greater than that obtained prior to operation. Baseline heart rate prior to operation was 60 ± 3 beats/min. After control recordings, 1.2 ± 0.2 liters of normal saline, warmed to 37°C, were infused over 5-10 minutes to raise left atrial pressure by 15 mm Hg. Data were averaged over 10 to 15-second periods, which included several respiratory cycles, for each 5 mm Hg increment in left atrial pressure. This was important, because many of the conscious dogs exhibited marked sinus arrhythmia. When left atrial pressure rose by more than 15 mm Hg, some dogs became restless and excited. Thus, the amount of saline infusion used in this study can be considered the maximum tolerated by the resting, conscious dog. When left atrial pressure rose by 15 mm Hg, isoproterenol, 0.4 /ug/kg per min, was infused for 5 minutes. This protocol was repeated several days later, substituting dobutamine (20 jiig/kg per min) for isoproterenol. On separate days, the inotropic agents were infused in the absence of volume loading. The effects of volume loading also were studied in four of the dogs with open chests after implantation of the electromagnetic flow probe. In these experiments, saline was infused at a rate similar to that used for the conscious dogs. In six other conscious dogs, volume loading was performed by infusing blood, maintained at 37°, over 5-10 minutes until 30 ml/kg had been infused and left atrial pressure increased by 15 mm Hg. In these dogs, on a different day, as well as in 10 other conscious dogs, volume depletion was studied by withdrawing blood at a rate of 0.5 ml/kg per min for 1 hour, until 30 ml of blood per kg had been withdrawn. Finally, the effects of severe exercise were examined with radiotelemetry of aortic blood flow from the unrestrained conscious dogs running spontaneously in the field at speeds of up to 25 mph behind a mobile recording unit. Data were recorded on a multichannel tape recorder and played back on a direct-writing oscillograph. A cardiotachometer, triggered by the signal from the pressure pulse, provided instantaneous and continuous records of heart rate. Mean arterial pressure and cardiac output were obtained by electronic resistance-capacitance filters with TABLE 1 Comparison of the Effects of Volume Loading in Conscious and Anesthetized Dogs Control 1978 +5 + 10 + 15 Cardiac output (ml/min) Conscious (n = 1) Anesthetized (n = 4) 2340 ± 80 2130 ± 170 3720 ± 150* 3450 ± 65* 4630 ± 180* 5050 ± 230* 5760 ±210* 6400 ± 73* Heart rate (beats/min) Conscious (n = 7) Anesthetized (n = 4) 62 ± 2 163 ± 14 100 ± 4* 146 ± 13t 125 ± 5* 149 ± 13t 155 ± 6* 157 ± 13t Stroke volume (ml) Conscious (n = 1) Anesthetized (n = 4) 37 ± 2 13 ± 2 38 ± 2 24 ± 2*t 37 ± 2 36 ± 5*t 38 ± 2 43 ± 9*t Mean arterial pressure (mm Hg) Conscious (n = 7) Anesthetized (n = 4) 84 ± 6 77 ± 6 122 ± 8* 115 ± 14* 129 ± 5* 113 ± 14* 101 ± 8* 95 ± 8* * Significantly different from control, P < 0.01. t Anesthetized response significantly different from conscious, P < 0.01. CONTROL OF CARDIAC OVTPVTIVatner and Boettcher AORTIC (LAi.™) ,Of " W _. • ! - - : - r J H | : D •• TS TSSF MEAN (mmHg) ] ^ ^ ^ ,. ^ g Downloaded from http://circres.ahajournals.org/ by guest on April 30, 2017 FIGURE 1 Effects of volume loading in a conscious dog are shown on responses of phasic and mean aortic blood flow (cardiac output), heart rate, calculated stroke volume, and mean arterial and left atrial pressures. In the conscious dog, volume loading increased cardiac output and heart rate but not stroke volume. 2-second time constants. Stroke volume was calculated as the quotient of cardiac output and heart rate and confirmed by means of an electronic integrator. Responses were compared to control by paired Mest.5 Values are reported as averages ± SEM. Results 1. Effects of Volume Loading (Table 1) In the conscious dogs, saline infusion elevated left atrial pressure by 15 mm Hg, cardiac output from 2340 ± 80 to 559 5760 ±210 ml/min (147 ± 7%), heart rate from 62 ± 2 to 155 ± 6 beats/min (143 ± 7%), and mean arterial pressure from 84 ± 6 to 129 ± 5 mm Hg, but failed to alter stroke volume from a control of 37 ± 2 ml (Fig. 1). Whereas stroke volume remained constant, there was a graded increase in heart rate in response to graded increases in left atrial pressure and volume loading (Table 1). When blood was infused into six other conscious dogs, cardiac output rose from 2460 ± 160 to 4900 ± 240 ml/ min (100 ± 5%), heart rate rose from 66 ± 5 to 133 ± 6 beats/min (108 ± 10%), and stroke volume fell, but not significantly, from a control of 38 ± 2 ml (Fig. 2). In four anesthetized dogs with the chest open, when atrial pressure was elevated by an amount equivalent to that induced in the conscious state, cardiac output rose from 2130 ± 170 to 6400 + 730 ml/min (208 ± 47%). In contrast to the results for conscious animals, the increases in cardiac output were mediated entirely by increases in stroke volume, which increased from 13 ± 2 to 43 ± 9 ml (243 ± 89%), while heart rate did not change significantly (Table 1; Fig. 3). 2. Effects of Inotropic Interventions Isoproterenol infusion without prior volume loading increased cardiac output by 102 ± 5% from a control of 2500 ± 230 ml/min and heart rate by 173 ± 16% from a control of 72 ± 5 beats/min; it reduced mean arterial pressure by 25 ± 2% from a control of 86 ± 8 mm Hg, stroke volume by 25 ± 3% from a control of 35 ± 3 ml (Fig. 4), and mean left atrial pressure by 4 ± 1 mm Hg. 36 AORTIC FLOW (L/min) SALINE INRISION 0 10 CARDIAC OUTPUT (L/min) 20C HEART RATE (beats/min) 0100 STROKE VOLUME (ml) 0200- MEAN ARTERIAL PRESSURE (mmHg) MEAN ATRIAL PRESSURE (mmHg) FIGURE 2 Effects of volume loading with blood (right of center) are compared with volume depletion (left of center) on responses of cardiac output (circles and solid lines), heart rate (triangles and dotted lines), and stroke volume (squares and dashed lines). Heart rate rose almost equally with volume loading and depletion. In contrast, stroke volume fell strikingly with hemorrhage and remained essentially constant with infusion of blood. 025- 0- FIGURE 3 Effects of volume loading in an anesthetized, openchest dog are shown on responses of aortic flow, cardiac output, heart rate, calculated stroke volume, and mean arterial and left atrial pressures. In contrast to the response in Figure I, in the anesthetized open-chest dog, volume loading increased cardiac output and stroke volume but not heart rate. CIRCULATION RESEARCH 560 -r FROM CONTROL -Wt- CARDIAC OUTPUT HEART RATE • [S3 O • SALINE BLOOD HEMORRHAGE ISOPROTERENOL ^ ISOPROTERENOL & S A L M • EXERCISE STROKE VOLUME Downloaded from http://circres.ahajournals.org/ by guest on April 30, 2017 FIGURE 4 Comparison of the effects of volume loading with saline (solid bars) and with blood (lined bars), volume depletion by hemorrhage (checked bars), isoproterenol infusion (stippled bars), isoproterenol plus volume loading (cross-hatched bars), and exercise (clear bars) on cardiac output, heart rate, and stroke volume. Note that only isoproterenol plus volume loading or exercise increased stroke volume, and in both cases by approximately 25%. Dobutamine infusion without prior volume loading increased cardiac output by 101 ± 7% from a control of 2270 ± 200 ml/min, heart rate by 61 ± 3 % from a control of 66 ± 2 beats/min, mean arterial pressure by 13 ± 6% from a control of 86 ± 5 mm Hg, and stroke volume by 25 ± 3% from a control of 36 ± 4 ml; it did not affect left atrial pressure significantly. 3. Effects of Combined Volume Loading and Inotropic Stimulation (Fig. 4) The superimposition of isoproterenol infusion in the volume-loaded state increased (from control) cardiac output by 355 ± 27%, heart rate by 191 ± 19%, mean arterial pressure by 61 ± 8 % , and stroke volume by 42 ± 2%, while left atrial pressure rose by an identical amount, 15 ± 1 mm Hg, as occurred with volume loading above. 4. Effects of Severe Exercise (Fig. 4) From reclining control values equivalent to those reported above under sections 1 and 2, exercise increased cardiac output by 402 ± 24%, heart rate by 312 ± 16%, and stroke volume by 27 ± 2%. A prior study from this laboratory has shown that increases in stroke volume are greater and increases in heart rate are less during exercise when control values are taken in the standing, as opposed to reclining, position.6 5. Effects of Volume Depletion (Fig. 2) After a hemorrhage of 30 ml/kg in 16 conscious dogs, there was a progressive fall in cardiac output from 2420 ± 90 ml/min to 1180 ± 90 ml/min (-49 ± 4%) and in stroke volume from 37 ± 2 to 8 ± 1 ml (-75 ± 2%), while heart rate rose from 70 ± 3 to 144 ± 8 beats/min (113 ± 15%). Discussion It is generally held that "an increased flow of blood from the veins into the heart automatically forces an VOL. 42, No. 4, APRIL 1978 equivalent increase in cardiac output by distending the ventricle and increasing stroke volume."7 Indeed, in the present study, in dogs anesthetized and with an open chest, the increased cardiac output observed with volume loading occurred entirely through increases in stroke volume (Fig. 3). This is consistent with findings of other investigators who used a similar preparaton.'- 8 " It would not have been surprising to find that volume loading increased cardiac output in the conscious dog as a result of increased in both heart rate and stroke volume, as has been observed previously.l0~12 However, we did not expect to find, in the conscious dog, that the maximum tolerable saline infusion did not result in any significant increase in stroke volume (Table 1); the rise in cardiac output was mediated entirely by tachycardia, which presumably was due to the Bainbridge reflex.l3~15 It is of interest that, while stroke volume remained constant, graded increases in volume loading and left atrial pressure induced graded reflex increases in heart rate in the conscious dog. It is important to point out that the reflex effects of volume loading are complex and are not due simply to effects on atrial stretch; they include activation of baroreceptor afferents15 and also may involve chemoreceptor afferents due to reductions in hematocrit induced by the saline infusion. However, similar results were observed with infusion of blood (Fig. 2), which indicates that chemoreceptor afferents stimulated by hemodilution with saline did not significantly modify the stroke volume response, i.e., stroke volume remained constant in the conscious dog despite a massive increase in preload. The finding that stroke volume remains constant under these conditions differs from those of previous studies in this field,8"12 including those conducted in conscious dogs.10"12 Since afterload rose with volume loading, it is possible that this was responsible, in part, for preventing a rise in stroke volume. However, this aspect of the response to volume loading was not unique to the present experiments in conscious dogs (Table 1) and. thus, cannot reconcile the differences between the results in this study in conscious and anesthetized dogs, or the differences between the results in the present and prior studies.""12 It is more likely that the differences can be reconciled on the basis of differences in baseline heart rate. In the present study, particular attention was paid to studying trained dogs with physiologically low heart rates, whereas previous studies on anesthetized preparations8' " or conscious dogs,10"12 all were conducted on animals with higher than normal heart rates. The average resting cardiac rate in the present study in conscious, instrumented dogs was 62 ± 2 beats/min, and this value is close to that of the resting normal dog without prior operation and instrumentation. Under these conditions, i.e., reclining conscious dogs with normal spontaneous rhythm and low physiological heart rates, stroke volume is relatively large at rest and cannot be elevated further by maximally tolerable volume loading. In another study from our laboratory"' it was observed that this amount of volume loading resulted in only a slight increase in end-diastolic ventricular dimensions,1" indicating that end-diastolic cardiac size is near maximal in the reclining conscious dog with a physiological heart CONTROL OF CARDIAC OUTPUT'/Vatner and Boettcher Downloaded from http://circres.ahajournals.org/ by guest on April 30, 2017 rate. Cowley and Guyton" also observed the importance of cardiac rate in achieving an increase in cardiac output when venous return was elevated by opening an arteriovenous fistula. Although the dogs in that study were anesthetized, their initial heart rates were low, due to surgically induced heart block.17 The experiments with hemorrhage indicate the extent to which stroke volume can be reduced in the conscious dog when preload is reduced. The magnitude of the reduction in stroke volume with hemorrhage further illustrates the relatively large baseline stroke volume in the resting, conscious, reclining animal (Fig. 2). To a great extent, the reduction in stroke volume is due to the reflex tachcardia induced by hemorrhage. It is of interest that similar amounts of volume depletion and loading (30 ml/ kg) induced comparable increases in cardiac rate (Fig. 2). Elevating the inotropic state by infusing isoproterenol also failed to elevate stroke volume in the present study. However, dobutamine, a sympathomimetic amine with a less positive chronotropic effect than isoproterenol, elevated stroke volume slightly, by approximately 25%. Thus, stroke volume can be increased slightly by administration of a positive inotropic agent, even when baseline heart rate is low. To achieve a greater effect on stroke volume, myocardial contractility was stimulated by isoproterenol and dobutamine infusions after volume loading. Even under these extreme circumstances, stroke volume rose only by 26% with isoproterenol and by 42% with dobutamine. This response approximated that observed during maximal exercise in dogs running at speeds over 20 mph in the field, when stroke volume rose by 27%. However, a greater increase in stroke volume is observed when the maximal response during exercise is compared to a control value in the upright posture rather than in the reclining state,6 since stroke volume falls and heart rate rises upon assuming the upright posture. These findings indicate that in the normal, conscious, reclining dog with all control mechanisms intact, reduction of cardiac output by volume depletion is mediated entirely by a reduction in stroke volume, but augmentation of cardiac output in response to an elevation in preload is not mediated by increases in stroke volume, as long as heart rate can increase, because stroke volume is relatively large at rest. However, when an increase in preload is 561 combined with inotropic stimulation, as occurs during exercise, there is a modest increase in stroke volume. In contrast, in the aroused or anesthetized dog, stroke volume is diminished under baseline conditions and then can increase in response to a variety of stimuli, even with a simple increase in preload. Thus, the baseline values for heart rate and stroke volume prior to experimentation, as well as the strikingly different responses of the conscious and anesthetized, open-chest animal to volume loading, must be considered in the interpretation of data from experiments designed to assess the effects of interventions on stroke volume and heart rate. References 1. Starling EH: The Linacre lecture on the law of the heart. Given at Cambridge, 1915. London, Longmans, Green, 1918 2. Rushmer RF: Constancy of stroke volume in ventricular responses to exertion. Am J Physiol 196: 745-750, 1959 3. Rushmer SF, Smith O, Franklin D: Mechanisms of cardiac control in exercise. Circ Res 7: 602-627, 1959 4. Vatner SF, McRitchie RJ, Braunwald E: Effects of dobutamine on left ventricular performance, coronary dynamics and distribution of cardiac output in conscious dogs. J Clin Invest 53: 1265-1273, 1974 5. Snedecor GW, Cochran, WG: Statistical Methods. Ames, Iowa, Iowa State University Press, ed 6. pp 1967, 91-98 6. Vatner SF, Franklin D, Higgins CB, Patrick T, Braunwald E: Left ventricular response to severe exertion in untethered dogs. J Clin Invest 51: 3052-3060, 1972 7. Vander AJ, Sherman JH, Luciano DS: Human Physiology. The Mechanisms of Body Function. New York, McGraw-Hill, 1975, p 249 8. Berglund E: The function of the ventricles of the heart. Acta Physiol Scand 33 (suppl 119): 3-36, 1955 9. Weber KT, Janicki JS, Reeves RC, Hefner LT, Reeves, TJ: Determinants of stroke volume in the isolated canine heart. J Appl Physiol 37: 742-747, 1974 10. Bishop VS, Stone HL, Horwitz LD: Effects of tachycardia and ventricular pressure on stroke volume in the conscious dog. Am J Physiol 220: 436-439, 1971 11. Bishop VS, Stone HL, Guyton AC: Cardiac function curves in conscious dogs. Am J Physiol 207: 677-682, 1964 12. Bishop VS, Peterson FD: Pathways regulating cardiovascular changes during volume loading in awake dogs. Am J Physiol 231: 854-859, 1976 13. Bainbridge FA: Influence of venous filling upon the rate of the heart. J Physiol (Lond) 50: 65-84, 1915 14. Horwitz LD, Bishop VS: Effect of acute volume loading on heart rate in the conscious dog. Circ Res 30: 316-321, 1972 15. Vatner SF, Boettcher DH, Heyndrickx GR, McRitchie RJ: Reduced baroreflex sensitivity with volume loading in conscious dogs. Circ Res 37:236-242, 1975 16. Boettcher DH, Vatner SF, Heyndrickx GR, Braunwald E: Extent of utilization of the Frank-Starling mechanism in control of cardiac performance in the conscious dog. Am J Physiol, Heart Circulatory Physiol (in press) 17. Cowley AW, Guyton AC: Heart rate as a determinant of cardiac output in dogs with arteriovenous fistula. Am J Cardiol 28: 321-325, 1971 Regulation of cardiac output by stroke volume and heart rate in conscious dogs. S F Vatner and D H Boettcher Downloaded from http://circres.ahajournals.org/ by guest on April 30, 2017 Circ Res. 1978;42:557-561 doi: 10.1161/01.RES.42.4.557 Circulation Research is published by the American Heart Association, 7272 Greenville Avenue, Dallas, TX 75231 Copyright © 1978 American Heart Association, Inc. All rights reserved. Print ISSN: 0009-7330. Online ISSN: 1524-4571 The online version of this article, along with updated information and services, is located on the World Wide Web at: http://circres.ahajournals.org/content/42/4/557.citation Permissions: Requests for permissions to reproduce figures, tables, or portions of articles originally published in Circulation Research can be obtained via RightsLink, a service of the Copyright Clearance Center, not the Editorial Office. Once the online version of the published article for which permission is being requested is located, click Request Permissions in the middle column of the Web page under Services. Further information about this process is available in the Permissions and Rights Question and Answer document. Reprints: Information about reprints can be found online at: http://www.lww.com/reprints Subscriptions: Information about subscribing to Circulation Research is online at: http://circres.ahajournals.org//subscriptions/