* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Homeostasis pH
Survey
Document related concepts
Organ-on-a-chip wikipedia , lookup
Expanded genetic code wikipedia , lookup
Homeostasis wikipedia , lookup
15-Hydroxyeicosatetraenoic acid wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Specialized pro-resolving mediators wikipedia , lookup
Transcript
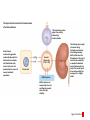
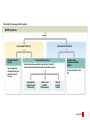
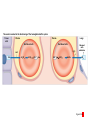
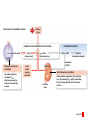
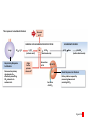
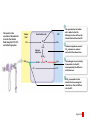
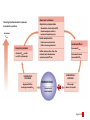
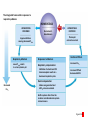
Homeostasis Section 2: Acid-Base Balance • Acid-base balance (H+ production = loss) – Normal plasma pH: 7.35–7.45 – H+ gains: many metabolic activities produce acids • • CO2 (to carbonic acid) from aerobic respiration Lactic acid from glycolysis – H+ losses and storage • • • Respiratory system eliminates CO2 H+ excretion from kidneys Buffers temporarily store H+ The major factors involved in the maintenance of acid-base balance The respiratory system plays a key role by eliminating carbon dioxide. Active tissues continuously generate carbon dioxide, which in solution forms carbonic acid. Additional acids, such as lactic acid, are produced in the course of normal metabolic operations. Normal plasma pH (7.35–7.45) Tissue cells Buffer Systems The kidneys play a major role by secreting hydrogen ions into the urine and generating buffers that enter the bloodstream. The rate of excretion rises and falls as needed to maintain normal plasma pH. As a result, the normal pH of urine varies widely but averages 6.0—slightly acidic. Buffer systems can temporarily store H and thereby provide short-term pH stability. Figure 24 Section 2 1 Section 2: Acid-Base Balance • Classes of acids – Fixed acids • Do not leave solution – • Remain in body fluids until kidney excretion Examples: sulfuric and phosphoric acid – Generated during catabolism of amino acids, phospholipids, and nucleic acids – Organic acids • Part of cellular metabolism – • Examples: lactic acid and ketones Most metabolized rapidly so no accumulation Section 2: Acid-Base Balance • Classes of acids (continued) – Volatile acids • • Can leave body by external respiration Example: carbonic acid (H2CO3) Module 24.5: Buffer systems • pH imbalance – ECH pH normally between 7.35 and 7.45 • Acidemia (plasma pH <7.35): acidosis (physiological state) – More common due to acid-producing metabolic activities – Effects » CNS function deteriorates, may cause coma » Cardiac contractions grow weak and irregular » Peripheral vasodilation causes BP drop • Alkalemia (plasma pH >7.45): alkalosis (physiological state) – Can be dangerous but relatively rare Figure 24.5 1 The narrow range of normal pH of the ECF, and the conditions that result from pH shifts outside the normal range The pH of the ECF (extracellular fluid) normally ranges from 7.35 to 7.45. When the pH of plasma falls below 7.5, acidemia exists. The physiological state that results is called acidosis. When the pH of plasma rises above 7.45, alkalemia exists. The physiological state that results is called alkalosis. Extremely acidic Extremely basic pH Severe acidosis (pH below 7.0) can be deadly because (1) central nervous system function deteriorates, and the individual may become comatose; (2) cardiac contractions grow weak and irregular, and signs and symptoms of heart failure may develop; and (3) peripheral vasodilation produces a dramatic drop in blood pressure, potentially producing circulatory collapse. Severe alkalosis is also dangerous, but serious cases are relatively rare. Figure 24.5 2 Module 24.5: Buffer systems • CO2 partial pressure effects on pH – Most important factor affecting body pH – H2O + CO2 H2CO3 H+ + HCO3– • Reversible reaction that can buffer body pH – Adjustments in respiratory rate can affect body pH The inverse relationship between the PCO2 and pH PCO2 40–45 mm Hg If PCO2 rises H2O CO2 H2CO3 H HCO3 When carbon dioxide levels rise, more carbonic acid forms, additional hydrogen ions and bicarbonate ions are released, and the pH goes down. pH 7.35–7.45 HOMEOSTASIS If PCO2 falls H HCO3 H2CO3 H2O CO2 When the PCO2 falls, the reaction runs in reverse, and carbonic acid dissociates into carbon dioxide and water. This removes H ions from solution and increases the pH. Figure 24.5 3 Module 24.5: Buffer systems • Buffer – Substance that opposes changes to pH by removing or adding H+ – Generally consists of: • Weak acid (HY) • Anion released by its dissociation (Y–) • HY H+ + Y– and H+ + Y– HY The reactions that occur when pH buffer systems function A buffer system in body fluids generally consists of a combination of a weak acid (HY) and the anion (Y) released by its dissociation. The anion functions as a weak base. In solution, molecules of the weak acid exist in equilibrium with its dissociation products. HY H Y Adding H to the solution upsets the equilibrium and results in the formation of additional molecules of the weak acid. H Y H H HY Removing H from the solution also upsets the equilibrium and results in the dissociation of additional molecules of HY. This releases H. H HY H Y H Figure 24.5 4 Module 24.5 Review a. Define acidemia and alkalemia. b. What is the most important factor affecting the pH of the ECF? c. Summarize the relationship between CO2 levels and pH. Module 24.6: Major body buffer systems • Three major body buffer systems – All can only temporarily affect pH (H+ not eliminated) 1. Phosphate buffer system • 2. Buffers pH of ICF and urine Carbonic acid–bicarbonate buffer system • • • Most important in ECF Fully reversible Bicarbonate reserves (from NaHCO3 in ECF) contribute Module 24.6: Major body buffer systems • Three major body buffer systems (continued) 3. Protein buffer systems (in ICF and ECF) • Usually operate under acid conditions (bind H+) – • Binding to carboxyl group (COOH–) and amino group (—NH2) Examples: – – – Hemoglobin buffer system » CO2 + H2O H2CO3 HCO3– + Hb-H+ » Only intracellular system with immediate effects Amino acid buffers (all proteins) Plasma proteins The body’s three major buffer systems Buffer Systems occur in Intracellular fluid (ICF) Phosphate Buffer System Has an important role in buffering the pH of the ICF and of urine Extracellular fluid (ECF) Carbonic Acid– Bicarbonate Buffer System Protein Buffer Systems Contribute to the regulation of pH in the ECF and ICF; interact extensively with the other two buffer systems Is most important in the ECF Hemoglobin buffer system (RBCs only) Amino acid buffers (All proteins) Plasma protein buffers Figure 24.6 1 BICARBONATE RESERVE The reactions of the carbonic acid–bicarbonate buffer system CARBONIC ACID–BICARBONATE BUFFER SYSTEM CO2 Lungs CO2 H2O H2CO3 (carbonic acid) Start H HCO3 (bicarbonate ion) Addition of H from metabolic activity Body fluids contain a large reserve of HCO3, primarily in the form of dissolved molecules of the weak base sodium bicarbonate (NaHCO3). This readily available supply of HCO3 is known as the bicarbonate reserve. HCO3 Na NaHCO3 (sodium bicarbonate) The primary function of the carbonic acid–bicarbonate buffer system is to protect against the effects of the organic and fixed acids generated through metabolic activity. In effect, it takes the H released by these acids and generates carbonic acid that dissociates into water and carbon dioxide, which can easily be eliminated at the lungs. Figure 24.6 4 The events involved in the functioning of the hemoglobin buffer system Tissue cells Plasma Lungs Plasma Red blood cells Red blood cells H2O H2O CO2 H2CO3 HCO3 Hb H Hb H HCO3 H2CO3 Released with exhalation CO2 Figure 24.6 2 Start The mechanism by free amino acids function in protein buffer systems Normal pH Increasing acidity (decreasing pH) (7.35–7.45) At the normal pH of body fluids (7.35– 7.45), the carboxyl groups of most amino acids have released their hydrogen ions. If pH drops, the carboxylate ion (COO) and the amino group (—NH2) of a free amino acid can act as weak bases and accept additional hydrogen ions, forming a carboxyl group (—COOH) and an amino ion (—NH3), respectively. Many of the R-groups can also accept hydrogen ions, forming RH. Figure 24.6 3 Module 24.6: Major body buffer systems • Disorders – Metabolic acid-base disorders • • Production or loss of excessive amounts of fixed or organic acids Carbonic acid–bicarbonate system works to counter – Respiratory acid-base disorders • • Imbalance of CO2 generation and elimination Must be corrected by depth and rate of respiration changes Module 24.6 Review a. Identify the body’s three major buffer systems. b. Describe the carbonic acid–bicarbonate buffer system. c. Describe the roles of the phosphate buffer system. Module 24.7: Metabolic acid-base disorders • Metabolic acid-base disorders – Metabolic acidosis • • Develops when large numbers of H+ are released by organic or fixed acids Accommodated by respiratory and renal responses – – Respiratory response » Increased respiratory rate lowers PCO2 » H+ + HCO3– H2CO3 H2O + CO2 Renal response » Occurs in PCT, DCT, and collecting system » H2O + CO2 H2CO3 H+ + HCO3– H+ secreted into urine HCO3– reabsorbed into ECF The responses to metabolic acidosis Start Addition of H CARBONIC ACID–BICARBONATE BUFFER SYSTEM CO2 CO2 H2O Lungs Respiratory Response to Acidosis Increased respiratory rate lowers PCO2, effectively converting carbonic acid molecules to water. H2CO3 (carbonic acid) Other buffer systems absorb H H HCO3 (bicarbonate ion) KIDNEYS BICARBONATE RESERVE HCO3 Na NaHCO3 (sodium bicarbonate) Generation of HCO3 Renal Response to Acidosis Secretion of H Kidney tubules respond by (1) secreting H ions, (2) removing CO2, and (3) reabsorbing HCO3 to help replenish the bicarbonate reserve. Figure 24.7 1 The activity of renal tubule cells in CO2 removal and HCO3 production Tubular fluid CO2 H H ECF Renal tubule cells Carbonic Na anhydrase CO2 H2O CO2 H2CO3 H HCO3 HCO3 Cl H Cl HCO3 Na Steps in CO2 removal and HCO3 production CO2 generated by the tubule cell is added to the CO2 diffusing into the cell from the urine and from the ECF. Carbonic anhydrase converts CO2 and water to carbonic acid, which then dissociates. The chloride ions exchanged for bicarbonate ions are excreted in the tubular fluid. Bicarbonate ions and sodium ions are transported into the ECF, adding to the bicarbonate reserve. Figure 24.7 2 Module 24.7: Metabolic acid-base disorders • Metabolic alkalosis – Develops when large numbers of H+ are removed from body fluids – – – Rate of kidney H+ secretion declines Tubular cells do not reclaim bicarbonate Collecting system transports bicarbonate into urine and retains acid (HCl) in ECF Module 24.7: Metabolic acid-base disorders • Metabolic alkalosis (continued) – Accommodated by respiratory and renal responses • Respiratory response – – • Decreased respiratory rate raises PCO2 H2O + CO2 H2CO3 H+ + HCO3– Renal response – – Occurs in PCT, DCT, and collecting system H2O + CO2 H2CO3 H+ + HCO3– » HCO3– secreted into urine (in exchange for Cl–) » H+ actively reabsorbed into ECF The responses to metabolic alkalosis Start Removal of H CARBONIC ACID–BICARBONATE BUFFER SYSTEM Lungs CO2 H2O Respiratory Response to Alkalosis Decreased respiratory rate elevates PCO2, effectively converting CO2 molecules to carbonic acid. H2CO3 (carbonic acid) Other buffer systems release H H HCO3 (bicarbonate ion) Generation of H BICARBONATE RESERVE HCO3 Na NaHCO3 (sodium bicarbonate) KIDNEYS Renal Response to Alkalosis Secretion of HCO3 Kidney tubules respond by conserving H ions and secreting HCO3. Figure 24.7 3 The events in the secretion of bicarbonate ions into the tubular fluid along the PCT, DCT, and collecting system Tubular fluid Renal tubule cells CO2 H2O CO2 ECF CO2 generated by the tubule cell is added to the CO2 diffusing into the cell from the tubular fluid and from the ECF. CO2 Carbonic anyhydrase converts CO2 and water to carbonic acid, which then dissociates. Carbonic anhydrase H2CO3 HCO3 HCO3 Cl H H Cl The hydrogen ions are actively transported into the ECF, accompanied by the diffusion of chloride ions. HCO3 is pumped into the tubular fluid in exchange for chloride ions that will diffuse into the ECF. Figure 24.7 4 Module 24.7 Review a. Describe metabolic acidosis. b. Describe metabolic alkalosis. c. lf the kidneys are conserving HCO3– and eliminating H+ in acidic urine, which is occurring: metabolic alkalosis or metabolic acidosis? CLINICAL MODULE 24.8: Respiratory acid-base disorders • Respiratory acid-base disorders – Respiratory acidosis • • • CO2 generation outpaces rate of CO2 elimination at lungs Shifts bicarbonate buffer system toward generating more carbonic acid H2O + CO2 H2CO3 H+ + HCO3– – – HCO3– goes into bicarbonate reserve H+ must be neutralized by any of the buffer systems » Respiratory (increased respiratory rate) » Renal (H+ secreted and HCO3– reabsorbed) » Proteins (bind free H+) The events in respiratory acidosis CARBONIC ACID–BICARBONATE BUFFER SYSTEM CO2 Lungs CO2 H2O H2CO2 (carbonic acid) When respiratory activity does not keep pace with the rate of CO2 generation, alveolar and plasma PCO2 increases. This upsets the equilibrium and drives the reaction to the right, generating additional H2CO3, which releases H and lowers plasma pH. H HCO3 (bicarbonate ion) As bicarbonate ions and hydrogen ions are released through the dissociation of carbonic acid, the excess bicarbonate ions become part of the bicarbonate reserve. BICARBONATE RESERVE HCO3 Na NaHCO3 (sodium bicarbonate) To limit the pH effects of respiratory acidosis, the excess H must either be tied up by other buffer systems or excreted at the kidneys. The underlying problem, however, cannot be eliminated without an increase in the respiratory rate. Figure 24.8 1 Responses to Acidosis The integrated homeostatic responses to respiratory acidosis Increased PCO2 Respiratory compensation Stimulation of arterial and CSF chemoreceptors results in increased respiratory rate. Renal compensation H ions are secreted and HCO3 ions are generated. Combined Effects Respiratory Acidosis Elevated PCO2 results in a fall in plasma pH Decreased PCO2 Buffer systems other than the carbonic acid–bicarbonate system accept H ions. Decreased H and increased HCO3 HOMEOSTASIS RESTORED HOMEOSTASIS DISTURBED Hypoventilation causing increased PCO2 HOMEOSTASIS Normal acidbase balance Start Plasma pH returns to normal Figure 24.8 2 CLINICAL MODULE 24.8: Respiratory acid-base disorders • Respiratory alkalosis – – CO2 elimination at lungs outpaces CO2 generation rate Shifts bicarbonate buffer system toward generating more carbonic acid H+ + HCO3– H2CO3 H2O + CO2 – • – H+ removed as CO2 exhaled and water formed Buffer system responses – – – Respiratory (decreased respiratory rate) Renal (HCO3– secreted and H+ reabsorbed) Proteins (release free H+) The events in respiratory alkalosis CARBONIC ACID–BICARBONATE BUFFER SYSTEM CO2 Lungs CO2 H2O H2CO2 (carbonic acid) If respiratory activity exceeds the rate of CO2 generation, alveolar and plasma PCO2 decline, and this disturbs the equilibrium and drives the reactions to the left, removing H and elevating plasma pH. H HCO3 (bicarbonate ion) BICARBONATE RESERVE HCO3 Na NaHCO3 (sodium bicarbonate) As bicarbonate ions and hydrogen ions are removed in the formation of carbonic acid, the bicarbonate ions— but not the hydrogen ions—are replaced by the bicarbonate reserve. Figure 24.8 3 The integrated homeostatic responses to respiratory alkalosis HOMEOSTASIS HOMEOSTASIS DISTURBED Start Normal acidbase balance HOMEOSTASIS RESTORED Plasma pH returns to normal Hyperventilation causing decreased PCO2 Respiratory Alkalosis Responses to Alkalosis Lower PCO2 results in a rise in plasma pH Respiratory compensation Inhibition of arterial and CSF chemoreceptors results in a decreased respiratory rate. Combined Effects Increased PCO2 Increased H and decreased HCO3 Renal compensation Decreased PCO2 H ions are generated and HCO3 ions are secreted. Buffer systems other than the carbonic acid–bicarbonate system release H ions. Figure 24.8 4 CLINICAL MODULE 24.8 Review a. Define respiratory acidosis and respiratory alkalosis. b. What would happen to the plasma PCO2 of a patient who has an airway obstruction? c. How would a decrease in the pH of body fluids affect the respiratory rate?































![ACID-BASE BALANCE Acid-base balance means regulation of [H + ]](http://s1.studyres.com/store/data/000604092_1-2059869358395bda26ef8b10d08c9fb9-150x150.png)






