* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Acid-Base Balance
Organ-on-a-chip wikipedia , lookup
Biomolecular engineering wikipedia , lookup
Homeostasis wikipedia , lookup
15-Hydroxyeicosatetraenoic acid wikipedia , lookup
Specialized pro-resolving mediators wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Genetic code wikipedia , lookup
Point accepted mutation wikipedia , lookup
Expanded genetic code wikipedia , lookup
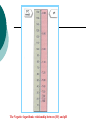
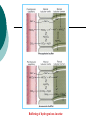
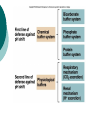
Acid-Base Balance Acid - Base Balance 1. 2. 3. Blood - normal pH of 7.2 – 7.45 < 7.2 = acidosis > 7.45 = alkalosis 3 buffer systems to maintain normal blood pH Buffers Removal of CO2 by lungs Removal of H+ ions by kidneys The Negative logarithmic relationship between [H+] and pH Acids are H+ donors. Bases are H+ acceptors, or give up OH- in solution. Acids and bases can be: Strong – dissociate completely in solution HCl, NaOH Weak – dissociate only partially in solution Lactic acid, carbonic acid The Body and pH Homeostasis of pH is tightly controlled Extracellular fluid = 7.4 Blood = 7.35 – 7.45 < 6.8 or > 8.0 death occurs Acidosis (acidemia) below 7.35 Alkalosis (alkalemia) above 7.45 Small changes in pH can produce major disturbances Most enzymes function only with narrow pH ranges Acid-base balance can also affect electrolytes (Na+, K+, Cl-) Can also affect hormones The body produces more acids than bases Acids take in with foods Acids produced by metabolism of lipids and proteins Cellular metabolism produces CO2. CO2 + H20 ↔ H2CO3 ↔ H+ + HCO3- Control of Acids 1. Buffer systems Take up H+ or release H+ as conditions change Buffer pairs – weak acid and a base Exchange a strong acid or base for a weak one Results in a much smaller pH change Bicarbonate buffer Sodium Bicarbonate (NaHCO3) and carbonic acid (H2CO3) Maintain a 20:1 ratio : HCO3- : H2CO3 HCl + NaHCO3 ↔ H2CO3 + NaCl NaOH + H2CO3 ↔ NaHCO3 + H2O Phosphate buffer Major intracellular buffer H+ + HPO42- ↔ H2PO4- OH- + H2PO4- ↔ H2O + H2PO42- Protein Buffers Includes hemoglobin, work in blood and ISF Carboxyl group gives up H+ Amino Group accepts H+ Side chains that can buffer H+ are present on 27 amino acids. 2. Respiratory mechanisms Exhalation of carbon dioxide Powerful, but only works with volatile acids Doesn’t affect fixed acids like lactic acid CO2 + H20 ↔ H2CO3 ↔ H+ + HCO3Body pH can be adjusted by changing rate and depth of breathing 3. Kidney excretion Can eliminate large amounts of acid Can also excrete base Can conserve and produce bicarb ions Most effective regulator of pH If kidneys fail, pH balance fails Hydrogen Ion Excretion in Kidney Buffering of hydrogen ions in urine Factors that increase or decrease H secretion and HCO3 Reabsorption by renal tubules : Increase in H ion secretion and HCO3 ion reabsorption PCO2 H , Decrease H ion secretion and HCO3 ion reabsorption PCO2 HCO3 H , HCO3 ECF volume ECF volume Angiotensin II Angiotensin II Aldosterone Aldosterone Hypokalemia Hyperkalemia Rates of correction Buffers function almost instantaneously Respiratory mechanisms take several minutes to hours Renal mechanisms may take several hours to days Buffers Protein Buffer Systems Amino Acid buffers Hemoglobin buffers Plasma Protein buffers Phosphate Buffer Systems Carbonic Acid – Bicarbonate Buffer System Buffer systems are used to keep the body in pH balance (homeostasis) It consists of a weak acid (H+)and its dissociation products (an anion) 3 major buffer systems in human Protein buffer system (includes hemoglobin buffer system) Regulates ICF & ECF (both plasma & interstitial fluid) Most important in ICF & hemoglobin Hemoglobin buffer system = carbonic anhydrase in RBC * it absorbs CO2 from ECF & get immediate effect Amino acids have carboxyl group (gives up H+) and Amino acids have amino group(can accept H+) Carbonic acid-bicarbonate buffer system Important in ECF Lots of carbon dioxide from metabolic acids It mixes with water & get carbonic acid which dissociates into H+ & HCO3Metabolic acids have H+ ; Our body has “bicarbonate reserve” Bicarbonate reserve = ample supply of bicarb in ECF These combine to form CO2 + H2O CO2 excreted via lungs Think of CO2 as an acid since it readily combines with water to become carbonic acid Maintenance of Acid-Base Balance Respiratory System: removal of CO2 by lungs – stabilizes the ECF, has direct effect on Carbonic Acid – Bicarbonate Buffer System Urinary System: removal of H+ ions by kidneys Regulation of blood pH by the respiratory system Kidney excretion of H+ Metabolic reactions produce nonvolatile acids One way to eliminate this huge load is to excrete H+ in urine In the proximal convoluted tubule, Na+/H+ antiporters secrete H+ as they reabsorb Na+ Intercalated cells of collecting duct include proton pumps that secrete H+ into tubule fluid; reabsorb K+ and HCO3Urine can be up to 1000 times more acidic than blood 2 other buffers can combine with H+ in collecting duct HPO42- and NH3 Secretion of H+ by intercalated cells in the collecting duct