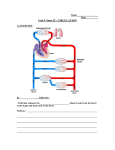
* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download growth and development
Cardiac contractility modulation wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Electrocardiography wikipedia , lookup
Cardiothoracic surgery wikipedia , lookup
Heart failure wikipedia , lookup
Coronary artery disease wikipedia , lookup
Antihypertensive drug wikipedia , lookup
Myocardial infarction wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Aortic stenosis wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Cardiac surgery wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Atrial septal defect wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
CONGENITAL HEART DISEASES Types. Acyanotic: VSD, PDA, ASD, AVSD, PS, CoA. Cyanotic: ToF, TGA. Types b. Right to left shunt: deoxygenated blood from the systemic venous system is being directed back into the systemic circulation without transiting the pulmonary vascular bed. c. All such defects are associated with the presence of a septal defect coupled with additional abnormalities which alter the pressure relationship between two sides of the heart. 1. Congenital malformations affecting the heart and/or the great vessels occur in a little under 1% of newborn infants. d. 2. i. pulmonary flow is reduced due to obstruction to normal flow into lung circulation and a septal defect behind obstruction through which blood may shunt from right to left, e.g. tetralogy of Fallot. Relative frequency of common congenital heart defects Defect Ventricular septal defect Persistent ductus arteriosus Atrial septal defect Pulmonary stenosis Aortic stenosis Coarctation of aorta Tetralogy of Fallot Transposition of great arteries Approximate frequency % 30 12 8 8 5 5 5 5 Three major subgroups exist: ii. bidirectional shunting is associated with very large communications between the left and right sides of the heart with free mixing of blood, e.g. single ventricle. iii. transposition of great arteries: the aorta and pulmonary artery are connected to the wrong side of the heart and as a result systemic venous blood is directed straight through into the systemic circulation again. 3. Acyanotic defects 4. The major presenting features are: a. Comprise 75% of all congenital heart defects. a. Presence of an abnormal murmur. b. ASD. Associated with an isolated left to right shunt, e.g. VSD, b. Development of symptoms or signs of congestive heart failure. c. Those which are not associated with any shunting, in which no septal defect is present, e.g. pulmonary stenosis. 4. Cyanotic defects a. Comprise 25% of all congenital heart defects. c. Central cyanosis. d. Any combination of the above. 1 Ventricular Septal Defect 1. f. If there is combination of PSM at LSE and MDM at apex and after 6 months, the MDM disappears, this indicates: Types: i. pulmonary hypertension is developing. a. Permembranous: defect is close to the membranous part of the ventricular septum. ii. VSD getting smaller. b. Subarterial: beneath the aortic and pulmonary valve. 4. Size of VSD and present signs c. Muscular: entirely enclosed in the muscular septum. Small + moderate VSD with L-to-R shunt 2. Symptoms: a. Can be asymptomatic. b. Dyspnoea with easy tiring. c. Failure to thrive. d. Recurrent chest infections. 3. Signs: Large VSD with L-to-R shunt Large VSD with bidirectional shunt + pulmonary hypertension. a. Loud harsh PSM at lower left sternal edge frequently associated with a thrill. b. P2 may be increased in pulmonary hypertension. c. MDM at apex due to increased flow through mitral valve. 5. Natural history a. Spontaneous closure: Acyanotic, asymptomatic. PSM. No P2. No MDM. Acyanotic but symptomatic. Hyperactive heart sounds. PSM. No P2. MDM at apex. Cyanosis. Quiet praecordium. No PSM. P2 loud. No MDM. i. variable rate. d. Atypical findings with a larger VSD: parasternal heave and displaced apex beat. ii. as high as 60% by 5th year of life. e. VSD need not have PSM since there is no flow if the heart contract and close the hole. iii. greatest number closed by 1 year, some by 5 year, few in adulthood. iv. large VSD unlikely to close after 2 years. 2 b. Complications: i. development of infundibular pulmonary stenosis to prevent too much blood from entering the lungs. ii. progressive aortic incompetence when one leaflet of the aortic valve prolapses into defect below valve. 7. Management a. Treatment of cardiac failure. b. Echocardiography and cardiac catheterization: i. to establish diagnosis. iii. subacute bacterial endocarditis: 30 – 40% mortality. ii. determine severity. iv. recurrent chest infection. iii. explore and confirm associated lesions. iv. if pulmonary flow:systemic flow > 2:1, then operate. v. Eisenmenger’s syndrome developing in adolescence due to rise in pulmonary vascular resistance leading to appearance of a right to left shunt with cyanosis. vi. congestive cardiac failure. c. Death: c. Pulmonary artery banding in infancy: Dammann-Muffler operation to decrease blood flow to lungs by 50%. d. Patch closure of VSD: i. done at 2 years. i. VSD die in infancy due to CCF or recurrent bronchopneumonia. ii. surgery after body weight is greater than 10kg. ii. 3% of VSD die later 1st year. e. SBE prophylaxis. iii. 90% of death occur in infancy. 6. Differentials a. PDA with pulmonary hypertension. b. AVSD. c. Infundibular pulmonary stenosis. b. Primum defects: low in the atrial septum and abut on the atrioventricular valves, which are abnormal and often incompetent. d. Truncus arteriosus. 2. Atrial Septal Defect 1. Classification a. Secundum defects: found in the fossa ovale. Symptoms 3 a. Generally asymptomatic in childhood. b. Not detected until 5 years of age. c. Severity of lesion depends on: i. site of defect. ii. pressure gradient across defect. d. Large defect may present early with easy tiring, dyspnoea and congestive cardiac failure. 3. Signs a. Fixed and wide splitting of soft S2. b. ESM at pulmonary area due to high pulmonary flow. c. Right ventricular hypertrophy. d. Atrial arrhythmias. 6. Associated defect a. Partial anomalous pulmonary-venous drainage usually with sinus venous which make ASD larger. b. Mitral valve prolapse. c. AVSD. d. Marfan syndrome. 7. Complications a. Congestive heart failure. c. Soft MDM at lower end of sternum, secondary to increased tricuspid flow. b. Recurrent chest infection. d. Palpable parasternal heave. c. Pulmonary hypertension. 4. Radiology d. SBE. a. Right heart enlargement. e. Paradoxical emboli: where emboli from right side of heart enters systemic circulation via ASD. b. Plethoric lung fields. c. Prominent pulmonary artery with small aorta. 5. ECG a. Right axis deviation. a. Cardiac catheterization only when pulmonary hypertension is present. b. Right bundle branch block. b. f. Atrial fibrillation and flutter. 8. Management Indication of operation: 4 i. symptomatic large heart with large shunt. d. ii. elevated pulmonary vascular resistance. i. includes a group of atrial septal defects low in thea trial septum which abut on the atrioventricular valves and may involve the upper part of the ventricular septum. c. Defect usually closed by direct suture, but may require insertion of a patch or a self-expanding ‘double umbrella device placed in the defect. d. Most require operation due to relatively safety of operation and possible complications if no operation is done. 9. Differentials a. Innocent murmur. b. Mild pulmonary stenosis. c. Partial AVSD. d. Aortic stenosis. Incomplete AVSD: ii. when the ventricular septum is intact only an atrial communication is present – ‘ostium primum ASD’ associated with malformations of the mitral and tricuspid valves which are often incompetent. e. Complete AVSD: commonly associated with Down’s syndrome. 2. Symptoms a. Early onset dyspnoea. b. Easy tiring. c. Feeding problems. Atrioventricular Septal Defect d. Failure to thrive. 1. e. Chest infections. f. Cardiac failure. g. Mild or no cyanosis. 3. Signs a. Split S2 with loud P2. b. ESM at pulmonary area. Anatomy a. AV septum is the septum between the right atrium and the left ventricle. b. AV septum consists of: i. anterior membranous part. ii. posterior muscular part. c. Shunting is from left ventricle to right atrium. 5 c. PSM. d. MDM at mitral area. 4. Radiology 2. Symptoms such as failure to thrive, dypsnoea and recurrent chest infections are similar to those of a large VSD. a. Plethoric lung fields. 3. Signs b. Right heart enlargement. a. Patients with a small PDA frequently remain asymptomatic. c. Left ventricular hypertrophy. b. Continuous machinery murmur audible at the upper left sternal border. 5. ECG a. Left axis deviation. c. In the presence of a large ductus, collapsing pulses are frequently apparent. b. Right bundle branch block. d. The apex may be displaced and forceful. 6. Management e. Presence of an apical mid-diastolic murmur. a. Treatment of cardiac failure in early infancy. 4. Differentials b. Surgery after cardiac catheterization: a. Venous hum. i. pulmonary artery bands. b. VSD with AR. ii. closure of VSD with repair of mitral valve. 5. Management Patent ductus arteriosus 1. b. In the small premature infant, delayed closure of the ductus often will occur after a period of weeks or months. a. In symptomatic premature infants, specific medical treatment with indomethacin, which inhibits prostaglandin synthesis, may be effective in promoting ductal constriction. Pathology a. Failure of the ductus arteriosus to close normally in the newborn period may be due to a congenital abnormality of the ductus or to severe prematurity. b. However, drug treatment is not effective in mature infants with a persistent ductus and in such patients, surgical intervention to close the ductus is indicated. 6 c. This should be carried out at an early stage in symptomatic patients but may be delayed until the second year of life. 4. Differentials of ESM a. ASD. b. Innocent murmur. c. MVP. 5. Differentials of thrill Pulmonary stenosis a. ASD. 1. Classification b. PDA. a. Valvular: 90% bicuspid / tricuspid. c. Coarctation or aorta. b. Subvalvular: 10% usually with Tetralogy of Fallot. 6. Radiology c. Supravalvular: includes peripheral arterial stenosis, e.g. in congenital rubella syndrome. a. Normal heart size. d. In such infants surgery is indicated to eliminate the risk of infective endocarditis. e. ‘Device closure’ by introduction of one or more embolization ‘coils’ or by placement of a ‘double umbrella device’ via a cardiac catheter. 2. May be asymptomatic, dyspnoeic or rarely cyanosis. b. Prominent pulmonary artery: abnormal convexity on the upper left heart border just below the aortic knuckle. 3. Signs 7. a. Parasternal heave. a. Mild pulmonary stenosis is a benign condition and is often not progressive. b. ESM best heard at pulmonary area and radiating through to the back and neck. c. Prognosis b. More severe pulmonary stenosis leads eventually to effort intolerance, angina on exertion and cardiac failure. Thrill at suprasternal notch. d. Ejection click at left sternal border: louder during expiration and fades on inspiration. c. Rarely, severe pulmonary stenosis may be present in early infancy with cyanosis due to right to left shunting through the foramen ovale or an associated ASD. e. 8. S2 split with soft P2. Management 7 a. Surgical valvotomy. b. Inflation of a balloon in the valve orifice to separate the fused commissures. b. Presentation may be delayed until late in childhood or adolescence. 5. Signs a. Diminished or absent femoral pulses. b. Radiofemoral delay, commonly right only. Coarctation of aorta 1. Pathology a. Narrowing of aorta, most commonly around origin of subclavian artery. c. Higher upper limb pressure than lower limb pressure by 20 mmHg. 6. Non-functional PDA Coarctation of Aorta b. A discrete stricture is present in the distal part of the aortic arch close to the site of the ductus arteriosus. a. Blood supply to lower body via collaterals: 2. Associated with: i. internal mammary artery to inferior epigastric artery. a. PDA. ii. anastomosis at shoulder girdle. b. Bicuspid aortic valve. iii. superior intercostal artery to 1st intercostal artery. c. Aneurysm of circle of Willis. b. Symptoms: d. Subaortic stenosis. i. asymptomatic. 3. Types ii. headache, dizziness, tinnitus, epistaxis, palpitations and insomnia. a. Functional PDA Non-functional PDA iii. limb. intermittent claudication, delayed wound healing in lower b. 4. Symptoms c. Signs: i. prominent carotid pulse. a. Development of severe cardiac failure in the newborn period, often in the second or third week of life. 8 ii. visible / palpable collaterals in interscapular region. iii. loud ESM at aortic area radiating to neck and mitral area. 7. Radiology a. Cardiomegaly. b. Pulmonary congestion. c. Abnormal aortic knuckle. d. Rib notching due to presence of enlarged intercostal arteries. b. Pulmonary stenosis. c. Right ventricular hypertrophy. d. Overriding aorta. 2. Pathology a. Important lesions are pulmonary stenosis and VSD. b. Presence of severe pulmonary stenosis associated with infundibular muscular obstruction with valvar hypoplasia and commissure fusion leads to elevation of right ventricular pressure. 8. Complications c. In most patients, the systolic pressure in the left and right ventricles is equal, but the marked resistance to ejection into the pulmonary circulation, due to the stenosis, produces right to left shunting into the aorta. a. Left ventricular failure. b. Aortic dissection. c. Subarachnoid haemorrhage due to ruptured berry aneurysm. 9. Treatment a. Cyanosis appears gradually during the early months of life or rarely in later childhood and is more obvious on crying or on exertion. a. Infuse prostaglandins E1. b. b. Treat heart failure. i. development of intermittent episodes of severe hypoxia and cyanosis. c. Surgery if symptomatic, re-stenosis. 3. Symptoms Hypoxic spells: ii. they may appear spontaneously or precipitated by stress or exercise. Tetralogy of Fallot 1. Components a. VSD. iii. loss of consciousness may occur. c. Failure to thrive. 9 d. Reduced exercise tolerance: children often adopt a squatting posture at regular intervals during exertion. d. Hypoxic spells. 4. Signs e. Relative anaemia. a. Single loud S2. f. Bleeding tendency: thrombocytopenia and prolonged PTT. b. ESM along left sternal edge and in pulmonary area and radiates through to the back: the softer the murmur, the more severe the PS. g. Hyperuricacemia: may cause acute renal failure. 8. Differentials 5. Pink Fallot a. Small VSD. a. signs. A less severe form, lesions not severe enough to cause b. Tricuspid atresia. 9. Associated lesions b. Left to right shunting. a. Right sided aortic arch (25%). c. Blood oxygen saturation in range of 92% with no cyanosis. b. Absent pulmonary or dysplastic valve (5%). 6. Course and prognosis c. Absent pulmonary arterial collateral circulation (5%). d. ASD. e. Pulmonary atresia. f. AVSD. 10. Radiology a. Normal, small or slightly enlarged heart or right ventricle. b. Large aorta, stenosis is usually infundibular. c. Diminished vascularity, oliguric lung fields. a. Cyanosis generally progresses gradually with diminishing exercise tolerance, finger clubbing and in severe cases, growth retardation. b. Development of cardiac failure is unusual, but the severe cyanosis leads to extreme compensatory polycythaemia and cerebral thromboembolic complications. 7. Complications a. Cerebral thrombosis due to increase in blood viscosity. b. Brain abscess normally due to embolization. c. SBE. 10 11. ECG a. RAD. b. RVH. c. Prolonged ventricular complex in older children. 12. Management a. Prophylactic propranolol: to clamp down sympathetic discharge to the heart and prevent cyanotic attack. b. Blalock – Taussic operation: creating a communication between the aorta and a pulmonary artery to increase pulmonary blood flow. 13. Case scenarios a. The management of choice is surgery when: i. child is old enough: > 2 years old. ii. pulmonary artery is of sufficient size (limiting factor). b. The timing of this procedure depends on the age of presentation and severity of disease. c. Severe FT in neonate / infant: i. provide immediate relief by increasing pulmonary blood flow. ii. IV PG E infusion in neonates to maintain patency of DA to provide pulmonary blood flow. c. Total correction: i. usually done when child is 3 – 4 years old. ii. mortality: 5 – 10%. iii. close VSD. d. Stable FT in neonate / infant: while awaiting for corrective surgery, conservative medical treatment is advisable. iv. ligate PDA. e. v. open PS. d. Conservative: i. prophylaxis against SBE. iii. BT shunt to link up subclavian artery to ipsilateral branch of pulmonary artery. FT in older children: total corrective surgery. Transposition of great arteries 1. Definition a. Atrial situs solitus. ii. dyspnoea and cyanosis: adopt knee-chest position and gives oxygen or morphine. b. AV concordant. iii. c. Ventriculoarterial disconcordant. correction of relative anaemia with Fe tablets. 11 d. Venoatrial concordant. c. Apart from the cyanosis the infant may appear completely normal. 2. Pathology 5. Signs a. Forceful right ventricular impulse at the left sternal edge. b. Narrowly split / single S2. c. Soft / absent systolic murmur. d. Signs of pulmonary hypertension / congestive heart failure. c. Survival is dependent on transfer of blood across from each circuit into the other via a foramen ovale, ductus arteriosus or a septal defect. 6. Differentials a. Respiratory distress syndrome. d. Affected infants generally survive for several days or even weeks due to shunting through the foramen ovale, but few live longer than a month without help. b. Persistent fetal circulation. c. Neonatal polyasplenia. 3. Associated problems d. Pulmonary atresia. a. PDA. e. Tricuspid atresia. b. VSD. f. Truncus arteriosus. c. Left ventricle outflow tract obstruction. g. Ebstein anomaly. 4. Symptoms h. Hypoplastic left heart. 7. Treatment a. The aorta and pulmonary arteries arise from the incorrect ventricles. b. Systemic venous blood is directed through from the right side of the heart back into the aorta and pulmonary venous blood through the left side of the heart and back into the pulmonary circulation. a. Cyanosis is present from the early hours of life and usually progresses gradually over the next few days. b. Metabolic acidosis also may develop if the situation persists untreated due to the tissue hypoxia. a. Cardiac catheterization is performed as an emergency procedure and a catheter with an inflatable balloon at the tip is passed into the left atrium via the foramen ovale. 12 b. After inflation of the balloon, the catheter is withdrawn with force into the right atrium producing a tear in the atrial septum and hence creating an atrial septal defect. c. This allows more effective interatrial shunting with amelioration of the cyanosis and hypoxia. c. Persistent truncus arteriosus 1. 8. Prone to arrhythmias. Pathology Surgery a. After successful balloon septostomy most infants will manage comfortably for many weeks or months. a. A rare defect associated with presence of a single artery which branches shortly after arising from the heart to give rise to the pulmonary artery and aorta. b. Surgical correction is now performed by transferring the pulmonary artery and the aorta back to their appropriate ventricles. b. The truncal valve usually sits astride a large VSD and receives blood from both right and left ventricles. c. This needs to be performed in early infancy, usually the first month of life, before the left ventricle has adapted to feeding the low pressure circulation. c. Pulmonary artery may be rudimentary and blood flow to lungs will be via bronchial artery. d. Mustard’s operation: wait till infant is 3 – 6 months old and then rerouting blood at atrial level by insertion of a complex intraatrial patch or baffle. e. Senning’s operation: repositioning the patient’s own atrial septum and infolding of part of the right atrium. 9. Corrected TGA a. Physiological correction (ventricle inversion). b. Anatomical and radiological transposition tell by: i. mitral / tricuspid valve. ii. incompetence of left AV valve. iii. subvalvular AS. 2. Symptoms a. toes. Central cyanosis: polycythaemia, clubbing of fingers and b. Breathlessness of exertion, fatigue. 3. Signs a. Poor physical growth. b. S2: loud, distinct and always single. c. Systolic murmur / thrill at base of heart. 4. Radiology a. Plethoric lung fields. 13 b. Cardiomegaly. c. Absence of pulmonary artery – concavity / vascular shadow of ventricle. 3. Signs a. Weak peripheral pulses. d. Enlarged supra-cardiac ‘aortic’ shadow. b. Triple rhythm. 5. Treatment 4. Radiology a. Surgical correction in early infancy. a. Oligemic lung fields. b. The pulmonary artery is separated from the truncus and after closure of the VSD leaving the aorta arising from the left ventricle a valved conduit is placed to connect the right ventricle to the pulmonary arteries. b. Gross cardiomegaly, enlarged right atrium – waterbag appearance (differential is pericardial effusion). 5. ECG a. Right atrial deviation. b. Pulmonary pulmonale. c. Frequently with partial / complete RBBB. 6. Treatment: usually arrhythmia control. Ebstein Anomaly 1. Pathology a. Downward displacement of a part of tricuspid valve into right ventricle. b. The posterior and medial cusps arise from the wall of the right ventricle instead of the annulus fibrosus resulting in downward displacement of right ventricle. Tricuspid atresia 1. c. May be associated with ASD. 2. Symptoms a. Breathlessness on exertion and fatigue. b. Central cyanosis during attacks of paroxysmal tachycardia. c. Development of CCF. Pathology a. The tricuspid valve is blocked completely and there is no communication between the right atrium and ventricle. b. Systemic venous blood passes via the foramen ovale or an ASD into the left side of the heart and at ventricular or arterial level a left to right shunt exists. 14 c. This allows blood to perfuse the pulmonary circulation, usually in reduced amounts. b. Blood in the right side of the heart passes via an ASD, foramen ovale or VSD into the left ventricle or aorta. 2. Signs c. The pulmonary circulation depends on collateral flow from the aorta via a PDA or other collateral channels. a. Cyanosis develops early. b. A systolic murmur is audible along the left sternal border. 3. ECG a. LAD. b. RAH. c. LVH. d. RV hypoplasia. 4. Treatment a. A palliative shunt operation may be performed in infancy. 2. Signs a. Cyanosis develops early. b. Continuous murmur due to associated PDA. 3. Treatment a. Prostaglandin infusion to maintain patency of the ductus. b. Early surgical treatment involves a systemic to pulmonary shunt procedure. c. Surgical correction by insertion of a ‘valved conduit’ from the right ventricle into the pulmonary arteries. b. Later in childhood, reconstructive cardiac surgery to create a wide anastomosis between the right atrium and the pulmonary arteries allowing systemic venous blood to pass directly into the pulmonary circulation (Fontan operation). Pulmonary atresia 1. Pathology a. The origin of the pulmonary artery from the right ventricle is completely obstructed or absent. 15