* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download MSTA WOW Chemistry
History of chemistry wikipedia , lookup
Acid–base reaction wikipedia , lookup
Photoredox catalysis wikipedia , lookup
Transition state theory wikipedia , lookup
Water pollution wikipedia , lookup
Green chemistry wikipedia , lookup
Liquid–liquid extraction wikipedia , lookup
Physical organic chemistry wikipedia , lookup
California Green Chemistry Initiative wikipedia , lookup
Process chemistry wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Hydrogen bond wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Electrochemistry wikipedia , lookup
Click chemistry wikipedia , lookup
Chemical reaction wikipedia , lookup
Safety data sheet wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Hydroformylation wikipedia , lookup
Atomic theory wikipedia , lookup
Nitrocellulose wikipedia , lookup
Catalytic reforming wikipedia , lookup
Stoichiometry wikipedia , lookup
Artificial photosynthesis wikipedia , lookup
Freshwater environmental quality parameters wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Industrial catalysts wikipedia , lookup
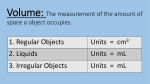
Put Some WOW in Chemistry with Demos MSTA, October 26, 2009 Janet Wise and Dennis Reed 1. 2. Density Adapted from http://www.stevespanglerscience.com/experiment/seven-layer-density-column Atomic Structure—The Black Box Ob-Scertainer Kit from Flinn, AP4549, $77.95 for set of 12 3. Old Foamey Kit from Flinn, AP2085, $17.91 4. Freezing Point Depression Concept: Observe the colligative property of freezing point depression by using rock salt to lower the freezing point of water. Materials: Crushed ice and rock salt Procedure: 1. Fill two 400-mL beakers with crushed ice. Add 5 mL of cold tap water to each beaker. 2. Stir the contents of each beaker with a stirring rod until both beakers are at a constant temperature, approximately one minute. 3. Measure the temperature of each beaker and record the readings. 4. Add 75 g of rock salt to one of the beakers. Continue stirring both beakers. Some of the salt will dissolve. 5. When the temperature in each beaker is constant, record the readings. 6. To clean up, flush the contents of each beaker down the drain with excess water. (Instructions from Glencoe's Chemistry: Matter and Change, 2002) 5. Variations on a Flame Test (Instructions from Flinn) A salt is dissolved in methanol. Upon combustion, a characteristic color is seen. Concept: Methanol is flammable and when mixed with oxygen and ignited creates quite an explosion. When an atom's electrons drop from the excited state to the ground state, a wavelength of light will be emitted. Metals have characteristic emissions of light that can be used to identify the individual metal, much like fingerprints can identify people. Materials: 1 gallon plastic milk jug with screw cap Methanol (methyl alcohol) Nitrate or chloride salt of Li, K, Ba, Sr, or Cu Meter stick with candle or fireplace match attached Safety Precautions: Methanol is toxic by ingestion and very flammable. Make sure all excess methanol is removed from the bottle. Cap the methanol bottle and remove it from demo area. Wear safety goggles. Procedure: 1. Make a concentrated solution of methanol and a salt. 2. Pour about 5 mL of the methanol solution into the milk jug and swirl to cover the sides of the jug. Open the jug and pour out excess methanol. Use a paper towel to remove excess methanol from the rim of the jug. This prevents flames that can melt the plastic. 3. Set the bottle on the counter, away from any flammable material. Dim the lights. Cover your ears! At an arm's length, bring a burning candle or match attached to a meter stick to a point about one inch above the mouth of the bottle. POW! 6. The Surprising Line Flinn Scientific, Inc., P.O. Box 219, Batavia, IL 60510-0219, 1-800-452-1261 Dennis Reed: [email protected] Janet Wise: [email protected] 7 Layer Density Column Objective: Create a stacked column of densities using liquids with known densities. Estimate the density of some unknown solids. Materials: Karo syrup or maple syrup Water Vegetable Oil Dawn dish soap (blue) Green Rubbing Isopropyl alcohol Lamp Oil Honey Graduated Cylinder Food Coloring Clear plastic cups Straws Glass beads Lead fishing sinkers Brass swivels Plastic beads Wood chips Cork Procedure: 1. Mix some water and food coloring in a plastic cup 2. If you lamp oil is green like the rubbing alcohol, then you need to add a different shade of food coloring to a small amount in a plastic cup. 3. Pour about 10 mL of Honey down the center of the graduated cylinder and try not to let it run down the sides. 4. Add 10 mL of Karo syrup or maple syrup to the graduated cylinder and again try not to let it run down the sides of the cylinder. 5. Now allow students to suggest which liquid they would like to go into the graduated cylinder. Pour each additional 10 mL of liquid in the cylinder by tilting the cylinder and allowing each to slowly run down the sides of the container. The slow pour is essential to prevent mixing of the liquids. 6. Allow time for the liquids to settle after all have been added to the graduated cylinder. 7. Give the students the density numbers for each of the liquids and let them label the liquid layers with density values on a drawing of the density column. 8. Find some solids small enough to drop in to the density column for students to estimate their densities as they settle in the liquid column. Data: Adapted from Material Dark Karo or maple syrup Light Karo syrup Water Gycerin Vegetable Oil Dawn (blue) Isopropyl Alcohol Lamp Oil Honey Baby Oil Density g/cm3 1.37 1.33 1.00 1.26 0.91 1.03 0.87 0.80 1.36 0.82 http://www.stevespanglerscience.com/experiment/seven-layer-density-column The Surprising Line "If the facts don't fit the theory, change the facts.—Albert Einstein Introduction: When precipitation reactions occur too fast and too easy, you could be missing a lot of good chemistry! Materials: Petri dishes (6—8 cm diameter) Potassium chromate (s) Distilled water Micro spatulas (Flinn #AP9015 Lead(II) nitrate (s) Overhead projector Potassium iodide (s) Safety Precautions: Lead nitrate is moderately toxic by ingestion; strong oxidant; a body tissue irritant; and a possible carcinogen. Potassium chromate is highly toxic by ingestion; harmful to skin, eyes, and respiratory tract; corrosive to body tissue; and a known carcinogen. Follow Flinn's safety guidelines for all chemical disposal. Wear chemical splash goggles, chemical-resistant gloves, and a chemical-resistant apron. Please consult Material Safety Data Sheets for further safety, handling, and disposal information. Procedure: 1. Place a thin layer of distilled water in a Petri dish and place the dish on a perfectly horizontal overhead projector. 2. Carefully add a small amount of the lead (Il) nitrate crystals into the water close to the side of the dish. 3. With a clean spatula, add solid potassium iodide to the exact opposite side of the Petri dish. 4. Watch carefully as a thin line appears and then grows. Repeat the procedure using lead (II) nitrate and potassium chromate. Discussion: Using this method to precipitate lead (II) iodide and lead (II) chromate allows students to analyze the process occurring. They can follow the dissolution, the migration of ions (related to size), and the precipitation reaction. Reference: deVos, Wobbe and Verdonk, A., "A New Road to Reactions," Journal of Chemical Education, August 1985, pp 648-9. Flinn Scientific Foundation 39 Old Foamey Chemistry Demonstration Kit Introduction Mix together hydrogen peroxide, sodium iodide solution, and dishwashing liquid in a tall cylinder and stand back. Your students will observe with amazement a catalyst in action as an enormous amount of soapy foam erupts from "Old Foamey!" Chemical Concepts • Catalysts • Decomposition Reactions Materials Needed (for each demonstration) Hydrogen peroxide, 30%, 20 mL* Sodium iodide solution, 2 M, 5 mL* Dishwashing liquid (Dove®), 10 mL* *Materials included in kit. Food coloring (optional) Graduated cylinder, 100-mL Graduated cylinder, 10-mL Plastic tray, several inches deep Safety Precautions Hydrogen peroxide, 30%, will act as an oxidizing agent with practically any substance. This substance is severely corrosive to the skin, eyes and respiratory tract; a very strong oxidant; and a dangerous fire and explosion risk. Do not heat this substance. Sodium iodide is slightly toxic by ingestion. Although the dishwashing liquid is considered non-hazardous, do not ingest the material. Do not stand over the reaction; steam and oxygen are produced quickly. Wear appropriate chemical splash goggles, chemical-resistant gloves and a chemical-resistant apron. This activity requires the use of hazardous components and/or has the potential for hazardous reactions. Please review current Material Safety Data Sheets for additional safety, handling, and disposal information. Procedure 1. Place a 100-mL graduated cylinder in a plastic tray that is several inches deep. 2. Measure out 20 mL of the 30% hydrogen peroxide into the 100-mL graduated cylinder. Caution: Wear chemical resistant gloves and goggles when handling 30% hydrogen peroxide. Contact with skin may cause burns. 3. Measure out 10 mL of dishwashing liquid into the 10-mL graduated cylinder and add it to the cylinder containing the hydrogen peroxide. Add a few drops of food coloring, if desired. Have your students observe that little or no reaction occurs. 4. Measure out 5 mL of sodium iodide solution using the 10-mL graduated cylinder. Quickly but carefully, add the sodium iodide solution to the 100-mL graduated cylinder. 5. Step back and observe the reaction. Disposal The foam and solution left in the cylinder may be rinsed down the drain with excess water. Tips You may want to do this demonstration in the laboratory sink since there is a lot of foam produced. Cleanup, however, is easy due to the presence of extremely safe products and the generous amount of detergent. IN2085 ™.. makes science teaching easier. 083io? • The cylinder will get hot, so let it cool before handling. • This demonstration can be easily and safely scaled up for larger audiences. A 500-mL or 1-L Pyrex® graduated cylinder works well in this case. You may have heard of this demonstration also referred to as elephant toothpaste. • The slight brown tinge of the foam at the beginning is due to the presence of free iodine produced by the extreme oxidizing ability of the 30% hydrogen peroxide. • Another catalyst that will catalyze this reaction is manganese (IV) oxide, MnOr • To demonstrate that oxygen is indeed one of the products, light a match and blow it out. Ideally, the center of the match will still be orange. Hold the match very close to the foam produced — the match should reignite. Discussion This demonstration evolves a good deal of heat as shown by the steam coming off of the foam as it is produced. The reaction, therefore, is exothermic. The action of a catalyst is demonstrated. The catalyst is the I-(aq) ion which speeds up the decomposition of the hydrogen peroxide. The decomposition of hydrogen peroxide produces steam and oxygen gas. The oxygen gas and water vapor cause the dishwashing liquid to foam. 2H202(aq)-->2H2O(g) + O2(g) + Energy Connecting to the National Standards This laboratory activity relates to the following National Science Education Standards (1996): Unifying Concepts and Processes: Grades K-12 Evidence, models, and explanation Content Standards: Grades 5-8 Content Standard B: Physical Science, properties and changes of properties in matter Content Standards: Grades 9-12 Content Standard B: Physical Science, structure and properties of matter, chemical reactions, interactions of energy and matter Answers to Worksheet Questions 1. Describe what happened in this demonstration. Hydrogen peroxide and dishwashing solution were added to a 100-mL graduated cylinder. A small amount of sodium iodide was added, which caused thick foam to erupt from the cylinder. 2. Write the chemical equation for the decomposition of hydrogen peroxide. 2H202(aq) -> 2H2O(g) + O2(g) 3. Why does the dishwashing solution foam? The water vapor and oxygen gas get trapped in the dishwashing liquid, causing it to foam. 4. What was the purpose of the sodium iodide? Was it consumed during the reaction? The sodium iodide served as a catalyst, which is a substance that speeds up a reaction but is not consumed during the reaction. It was not consumed during the reaction. Acknowledgement Special thanks to Jim and Julie Ealy of The Peddie School in Hightstown, NJ. Reference Stone, C. H. J. Chem. Ed. 1944, 21, 300. Materials for Old Foamey — Chemical Demonstration Kit are available from Flinn Scientific, Inc. Catalog No. Description AP2085 Old Foamey — Chemical Demonstration Kit Consult your Flinn Scientific Catalog/Reference Manual for current prices. -2© 2007 Flinn Scientific, Inc. All Rights Reserved. IN2085