* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Dec. 5 - The atom
Matter wave wikipedia , lookup
Elementary particle wikipedia , lookup
James Franck wikipedia , lookup
Delayed choice quantum eraser wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Double-slit experiment wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Chemical bond wikipedia , lookup
Geiger–Marsden experiment wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Wave–particle duality wikipedia , lookup
Ultrafast laser spectroscopy wikipedia , lookup
Atomic orbital wikipedia , lookup
Tight binding wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Electron configuration wikipedia , lookup
Hydrogen atom wikipedia , lookup
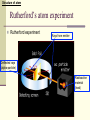
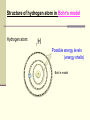
December 5, 2016 The Atom Why are there so many different materials in the world? Today’s Keywords (chemical) Element, molecule, atom; Electron, nucleus, Bohr atom; Photon, spectrum, spectroscopy Contents n Introduction n The atomic structure n When matter meets light n The laser – practical application 1. Introduction - Historical review on discovering the smallest pieces Introduction The smallest pieces by Democritus n Democritus, in about 530 BC, philosophically argued about a piece that could not be divided further, and named the smallest piece the “atom”. n The atom defined by Democritus: “uncuttable”, eternal and unchanging Introduction The smallest pieces by Dalton n In 18th century many chemists knew most materials can be broken down into simpler chemicals, and finally John Dalton (1766-1844) recognized that there are a few materials could not be broken down into other substance by any chemical means è The chemical materials called elements Example of (chemical) elements: Iron Gold Sodium Magnesium . . . Introduction The smallest pieces by Dalton n In 18th century many chemists knew most materials can be broken down into simpler chemicals, and finally John Dalton (1766-1844) recognized that there are a few materials could not be broken down into other substance by any chemical means è The chemical materials called elements. n Dalton suggested that for each chemical element there was a corresponding species of individual objects Example of atoms: è atoms Iron atom Gold atom Sodium atom Magnesium atom … Introduction The smallest pieces by Dalton n In 18th century many chemists knew most materials can be broken down into simpler chemicals, and finally John Dalton (1766-1844) recognized that there are a few materials could not be broken down into other substance by any chemical means è The chemical materials called elements. n Dalton suggested that for each chemical element there was a corresponding species of individual objects è atoms n Two or more atoms can be stuck together to make up most of the different kinds of material we see around us è molecules Introduction Element, molecule, and atom with an example: water n Water: a kind of material we can see around us; a chemical compound composed of two kinds elements hydrogen, oxygen: fundamental chemical elements n Hydrogen atom (H), oxygen atom (O): atoms n Water molecule: H2O (2 Hydrogen atoms + 1 oxygen atom) Introduction n However, No matter how persuasively argued, philosophical speculations on the existence of atoms were just speculations guess, assumption, suggestion Introduction Discovering chemical elements n In 19th century, a new process using electrical current, called electrolysis, was invented by Volta to break molecules down into atoms <Simple example of electrolysis> Electrolysis of water 2H2O(liquid) → 2H2(gas) + O2(gas) Water molecule Hydrogen gas molecule Oxygen gas molecule Introduction Discovering chemical elements (cont’d) n After the new process was developed, Dmitri Mendeleev invented a periodic table of the elements in 1869 for several dozen elements on the basis of their weights and by groups with distinctive chemical properties, although he could not know much about the structure of atoms The periodic table of the elements Period Group The colors represent different categories of elements. Please refer to wikipedia. Introduction Discovering chemical elements (cont’d) n Today, the periodic table lists 118 elements, of 92 appear in nature and the rest produced artificially n Many natural systems are constructed from just a few 99% of Earth’s solid mass: oxygen, silicon, magnesium, iron, aluminum, and calcium Most of human body atoms: hydrogen, carbon, oxygen, or nitrogen Most stars: the lightest atom, “hydrogen” The periodic table of the elements n The pattern of the elements in the periodic table displays a concentric arrangement of electrons into shells Period 1: One shell Period 4: four shells The periodic table of the elements n The pattern of the elements in the periodic table displays a concentric arrangement of electrons into shells Period 1: One shell Period 4: four shells ** Details will be discussed in “Atoms in combination” 2. The structure of the atom Structure of atom Atomic nucleus n In 1897, Joseph J. Thomson identified a much smaller and lighter particle than even the smallest atom known, which has a negative electrical charge è electron n Thomson argued that atoms are not the fundamental building blocks of matter, but rather are made up of things that are smaller and more fundamental, because except there is no place where the electron could come other than inside the atom Structure of atom Atomic nucleus – Rutherford exp. n In 1911, Ernest Rutherford (1871-1937) discovered the structure of atom using experiment started with a piece of radioactive material ** Radioactive material: matter that sends out energetic particles Structure of atom Rutherford’s atom experiment n Rutherford experiment Rays from emitter Deflected rays (alpha-particle) Radioactive material (lead) Structure of atom Atomic nucleus – Rutherford exp. n Rutherford found that a large part of each atom’s mass is located in a very small, compact object at the center. è nucleus n Later on, it was discovered that the nucleus itself is made up primarily of two different kinds of particles. è electrically positively charged proton, neutral neutron nucleons Structure of atom Rutherford atom • Rutherford’s atom model Electrons in orbits Structure of atom Rutherford atom • Rutherford’s atom model n The atom’s structure by Rutherford: A small dense positively charged nucleus is sitting at the atom’s center, with light negatively charged electrons “in orbit” circling it, like planets orbiting the Sun. Structure of atom Rutherford atom • Rutherford’s atom model n The atom’s structure by Rutherford: l a e r esitting at A small dense positively charged nucleus is h t n i a l p x e t ’ n the atom’s center, witholight negatively charged electrons d l u c l e d ! o m m o “in orbit” circling it, like planets orbiting the Sun. s t i a h e T h t è f o s r o i v a h e b Structure of atom Bohr’s atom model n In 1913, Niels Bohr (1885 -1962) produced a very strange theoretical model of the atom, which doesn’t match with our intuition about the real world, but it explained all the behaviors of atoms! Structure of atom Bohr’s atom model (cont’d) n His insight began with the fact that hot hydrogen atoms give off light in several separate wavelengths, rather than a continuous range of wavelengths. à He realized that circling electrons around the nucleus could not maintain their orbits at just any distance from the center. èHe suggested there were certain orbits in which an electron could exist for long periods of time without giving off radiation: electron energy levels or electron shells ** How to read element symbol n Element, element symbol, atomic number Helium: 2He # of nucleons: atomic mass (A) 4 2 He # of protons: identify a chemical element = atomic number (Z) Atomic mass A = atomic number Z + neutron number N of the atom Structure of hydrogen atom: 1 proton in nucleus + 1 electron Hydrogen atom: 1 - H + Structure of hydrogen atom in Rutherford’s model Hydrogen atom: 1 - H + Rutherford’s model Structure of hydrogen atom in Bohr’s model Hydrogen atom: 1 H Possible energy levels (energy shells) - + Bohr’s model Structure of atom Bohr atom n Ground state: (a) lowest energy level which electrons can occupy n Excited states:(b) and (c) all energy levels above the ground level 3. When matter meets light n We have learned, light = electromagnetic waves n In this section we will learn another side of light, light = particles (photons) When matter meets light Photons: Particles of light Assume that an electron is in an exited state. What will happen to extra energy when the electron moves to the lowest state? n The left over energy when the electrically charged electron moves from a high state to a lower state is emitted in the form of a single packet of electromagnetic radiation è a photon When matter meets light Photons: Particles of light (cont’d) n Whenever an electron jumps from a high to a lower energy level, a photon emits at the speed of light. (Go back to the figure of Bohr model. Electrons can exist in energy state1(=shell1), state2(shell 2), and so on.) When matter meets light Photons: Particles of light (cont’d) **Absorption of light is something like a mirror image of light emission: If a photon, with just the right amount of energy, meets matter, the photon can be absorbed and the electron will be pushed up to an excited state. When matter meets light Quantum leap Two key ideas embedded in Bohr’s atom model n When an electron moves from one allowed state to another, it cannot be at any place in between è quantum jump or quantum leap n The energies emitted in the two different jumps will generally be different from each other, but the sum of the two energies will equal that of the single large jump Structure of atom Bohr atom n Ground state: (a) Lowest energy level which electrons can occupy n Excited states: (b) Quantum leap to energy Level 3 by absorbing a photon (c) Quantum leap to energy level 2 by emitting a photon When matter meets light Quantum leap: an example *Quantum leaps are very much in evidence in our everyday life. (HW) *An example of quantum leaps: In fluorescence, n the atom absorbs a higher-energy photon of ultraviolet radiation. n Then, the atom emits two lower-energy photons, at least one of which is in the visible range. n Consequently, by shinning ultraviolet “black light” on the fluorescent material, it glows with a bright color. When matter meets light Spectrum Different atoms give off different characteristic photons n Different nuclei have different numbers of protons, so electrons circling them are in different energy levels When matter meets light Spectrum (cont’d) n Each chemical element emits a distinct set of characteristic photons à The total collection of photons emitted by a given atom is called its spectrum à Each possible quantum jump corresponds to light at a specific wavelength, so each type of atom produces a set of lines When matter meets light Different atoms give off different characteristic photons Each possible quantum jump corresponds to light at a specific wavelength, so each type of atom produces a set of lines Sodium 11Na Lithium 3Li When matter meets light Different atoms give off different characteristic photons • Elements impart distinctive colors to a flame Sodium 11Na Copper 29Cu Lithium 3Li When matter meets light Spectroscopy Spectroscopy n The study of forming and looking at spectra using devices such as spectrometers and spectroscopes n Astronomers use emission spectra to find the chemical composition of distant stars. n Spectroscopic analysis is used in manufacturing to search for impurities on production lines. 4. The laser – practical application of Bohr atom model The laser The laser n LASER: Light Amplification by Stimulated Emission of Radiation n Bohr atom provides an excellent way of understanding the workings of the laser The laser The process of stimulated emission: A. stimulated emission B. Light emitted by another atom C. In a laser, cascade stimulations happen very quickly The crests of all waves line up exactly C www.daviddarling.info/encyclopedia/ The laser The laser The process of stimulated emission: A. If a single photon of the correct frequency enters the system of excited states of atoms, it will pass the first atom and stimulate the emission of a second atom A Atom in excited state B Photon with the correct frequency stimulate the second atom to emit a photon with the same frequency The laser The laser The process of stimulated emission: B. The two photons with the same frequency of the excited atom state, then, will encounter other atoms, then will stimulate emission A Atom in excited state B Photon with the correct frequency stimulate the second atom to emit a photon with the same frequency The laser The laser The process of stimulated emission: C. This cascade stimulation happens very quickly, then, soon there is a flood of photons in one direction C A flood of photons with same frequency in one direction The laser Applications of lasers (HW) n Low-power lasers - optical scanners, light pointers, light measures n Finely focused laser beams - eye surgery (LASIK, LASEK, …) n Powerful lasers - cutting tools in factory, futuristic energy beam weapons (Star Wars laser sword?) , and so on. Next topic is, Quantum mechanics: chapter 5 www.sci.hokudai.ac.jp/~epark/ekpark/jos.html