* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Organic Chemistry IB Organic Chemistry 2016
Cracking (chemistry) wikipedia , lookup
Asymmetric induction wikipedia , lookup
Aromaticity wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Hydroformylation wikipedia , lookup
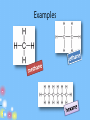
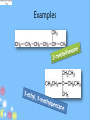
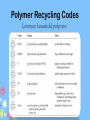
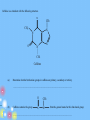
IB Organic Chemistry Organic – What is it? • Originally defined as the chemistry of all living things • Now defined as the chemistry of carbon and its compounds – Includes • All biological molecules • Fossil fuels • Most synthetic materials – plastics Homologous Series • There are so many organic molecules that we need a classification system • Homologous series : a family of like organic molecules – Members of the series have differing numbers of carbon atoms – Have similar characteristics and functional groups Series: Alkanes • • • • Carbon chains that have only single bonds Saturated hydrocarbons CH4 Methane Name ends in -ane C2H6 Ethane CnH2n+2 C3H8 Propane C4H10 Butane C5H12 Pentane Naming: IUPAC • International Union of Pure and Applied Chemistry • Rule 1: Identify the longest straight or continuous chain of carbon atoms •This identifies the stem or root of the name Number of Carbons Stem for IUPAC name 1 2 3 4 5 6 methethpropbutpenthex- Examples Naming: IUPAC Type Alkane Alkene Alkyne suffix -ane -ene -yne • Rule 2: add a suffix based on the type of chain The location of the double or triple bonds need to be specified Examples Naming: IUPAC • Rule 3: Name the side chains as the prefix of the name (label the carbon that it is located on) Number of Carbons Branch name 1 2 3 4 5 6 methylethylpropylbutylpentylhexyl- Examples Series: Alkenes • • • • Carbon chains that contain a double bond Unsaturated hydrocarbons Name ends in -ene C2H4 Ethene CnH2n C3H6 Propene C4H8 1-Butene C5H10 1-Pentene Series: Alkynes • • • • Carbon chains that contain a triple bond Unsaturated hydrocarbons Name ends in -yne C2H2 Ethyne CnH2n-2 C3H4 Propyne C4H6 1-Butyne C5H8 1-Pentyne Functional Groups • Groups attached to the carbon framework that give the molecule certain characteristics Series: Alcohols • Functional Group: hydroxyl group, -OH • Name ends in -ol CH3OH Methanol CH3CH2OH Ethanol CH3CH2CH2OH 1-Propanol Series: Carboxylic acids • Functional Group: carboxyl group, -COOH • Name ends in –oic acid No numbers needed! HCOOH Methanoic acid CH3COOH Ethanoic acid CH3CH2COOH Propanoic acid Series: Aldehyde • Functional Group: carbonyl group • Name ends in -al No numbers needed! H CH2O Methanal CH3CHO Ethanal CH3CH2CHO Propanal Series: Ketone • Functional Group: carbonyl group • Name ends in -one Numbers needed when > 4 carbons CH3COCH3 Propanone CH3COCH2CH3 Butanone Series: Esters • Functional Group: -COOR • Name ends in –oate Propyl ethanoate CH3COOCH3 Methyl ethanoate CH3COOCH2CH3 Ethyl ethanoate CH3CH2COOCH3 Methyl propanoate CH3CH2COOCH2CH3 Ethyl propanoate Series: Halides • Functional Group: presence of halogen • Name – suffix stays the same CH3CH2CH2Cl 1-chloropropane • Empirical Types of Formulas (simplest ratio) • Molecular • Full Structural • Condensed Structural • Line formula Naming: IUPAC • International Union of Pure and Applied Chemistry • Rule 1: Identify the longest straight or continuous chain of carbon atoms •This identifies the stem of the name Number of Carbons Stem for IUPAC name 1 2 3 4 5 6 methethpropbutpenthex- Naming: IUPAC • Rule 2: Use the functional group ending as the suffix of the name (-ane, -ene, -yne, ol, -al, -one, -oic acid, -oate) • Rule 3: Name the side chains as the prefix of the name (label the carbon that it is located on) Side chain -CH3 Prefix methyl -C2H5 -C3H7 -F, -Br, -I, -Cl ethyl propyl fluoro-, chloro-, iodo-, bromo- -NH2 -OH aminohydroxy- ID ONLY • CH3-O-CH2-CH3 • CH3—CH2—NH2 methoxyethane (ether) ethylamine (amine) • CH3—CH2—C—NH2 Propanamide (amide) O Review Activity • Part I – Come up with 10 names and 10 structures…. – Place them in random order • Part II – Switch papers with someone else and solve • Part III – Check each others answers – Check another group’s answers if time… Rings • Add the word cyclo- to the stem • Examples – cyclopropane – cyclobutane – cyclopentane – cyclohexane Aromatic Molecules • Contain the benzene ring • Unsaturated cyclohexene Isomers • Molecules that have the same kinds and numbers of atoms, but different arrangements 2-methylpropane CH3CHCH3 CH3 butane CH3CH2CH2CH3 Cis-Trans Isomers • • • • Alkenes Rotation at c-c double bonds restricted cis = same side of double bond trans = opposite sides Primary, Secondary, & Tertiary • Primary carbon atoms: attached to functional group and 1 other carbon • Secondary carbon atoms: attached to the functional group and 2 carbons • Tertiary carbon atoms: attached to functional group and 3 other carbons Activity 1. Determine the strongest IMF present for: – Alcohols – halides – Ketones – Aldehydes – C. acids – alkanes 2. Determine the polarity. (high/medium/lo w) 3. Cut out and arrange your pieces by physical properties… Physical Properties • Boiling point: As the carbon chain lengthens, the boiling point increases • Solubility: – Length of hydrocarbon chain: • chains are nonpolar • Solubility decreases as chain increases – Functional group: ability to form hydrogen bonds and interact with water • Alcohols, aldehydes, ketones, and carboxylic acids soluble • Halogenalkanes are not soluble in water Halogenoalkanes… • Very slightly soluble in water. • Only slightly polar • Do not effectively break the hydrogen bonds between water molecules. • Soluble in nonpolar or less polar organic solvents such as alcohol and benzene . Physical Properties • Volatility: ease of changing into the gaseous state 1. Chain length: • • • As the chain increases, the boiling point increases: Smaller chains likely to be gases or liquids Larger chains likely to be solids Physical Properties • Volatility 2. Branching of the chain: • more branches = more space around the molecule = weaker IMFs = lower boiling points 3. Functional groups: • Polar groups have higher boiling points- stronger intermolecular forces- harder to break apart Most volatile Least volatile Increasing boiling points Increasing strength of intermolecular attraction Alkane Reactivity • Low reactivity! C-C and C-H bonds are very strong • Combustion – Complete produces carbon dioxide and water – Incomplete limited oxygen – new products formed Carbon monoxide (limited oxygen) Carbon (extremely limited oxygen) Alkane Combustion • Carbon dioxide – greenhouse gas • Water – greenhouse gas (absorb radiation and contribute to global warming) • Carbon monoxide – toxic to us (absorbed by the body and bonds to hemoglobin preventing oxygen from being carried) • Carbon – particulates formed in the air, and is harmful to respiratory system Alkane Reactivity • Substitution reactions – main type of reaction alkanes undergo – An incoming species takes the place of a H atom – Often called free radical substitutions Cl2 + CH4 CH3Cl + HCl Three Basic Steps in a Free Radical Mechanism 1. Chain initiationThe chain is initiated by UV light breaking a chlorine molecule into free radicals. Cl2 2Cl. 39 Homolytic Fission • Free radicals are formed if a bond splits evenly - each atom getting one of the two electrons. The name given to this is homolytic fission. 40 Three Basic Steps in a Free Radical Mechanism 2. Chain propagation reactions- These are the reactions which keep the chain going Have to have free radicals in the system to keep the chain going CH4 + Cl. CH3. + HCl CH3. + Cl2 CH3Cl + Cl . 41 Three Basic Steps in a Free Radical Mechanism 3. Chain termination reactions These are reactions which remove free radicals from the system without replacing them by new ones – terminating the rxn 2 Cl. Cl2 CH3. + Cl. CH3Cl CH3. + CH3. CH3CH3 42 Alkene Addition Rxns • Moves from unsaturated to saturated • Hydrogenation – addition of hydrogen on the double bond – Margarine is solid at room temperature because it has been hydrogenated and is saturated (higher melting points) Alkene Addition Rxns • Halogenation: occur at room temperature – Accompanied by the loss of color of the reacting halogen Alkene Addition Rxns • Hydrohalogenation: occur at room temperature Alkene Addition Rxns • Hydration – Addition of water turns the alkene into an alcohol Water splits into H and OH Markovnikov’s Rule • Hydrogens add to the carbon that has the most hydrogens already! Polymers • Types of synthetic and natural polymers. Polymerization of Alkenes • Alkene acts as the monomer (building block of a polymer) • Plastics are polymers of alkenes polyethene polypropene Polymerization of Alkenes -PVC • Polymerization of chloroethene into polychloroethene – aka: PVC (polyvinylchloride) Polymer Recycling Codes Common household polymers Polymers • The number code indicates the polymer type Alcohols • Increased solubility in water • CnH2n+1OH • Oxidation: – Complete oxidation: combustion reactions 2CH3OH(l) + 3O2(g) 2CO2(g) + 4H2O(g) Alcohols • O and heat can be added to oxidize the OH group (in the form of Potassium dichromate VI) • Primary alcohols-oxidized to carboxylic acids • 2ndary alcohols- oxidized to ketones • Tertiary alcohols- NR Oxidation • Alcohols are oxidized to alkanals (aldehydes) or alkanones (ketones) Oxidation: Primary Alcohols • Ethanol is oxidized to ethanal Oxidizing agent: Potassium dichromate VI • Can be further oxidized to ethanoic acid • Wine left exposed to air starts to smell of vinegar – this is the ethanoic acid Oxidation: Secondary Alcohols • Propanol oxidized to propanone Oxidation: Tertiary Alcohols • There is no hydrogen attached to the tertiary carbon. It is not possible for the tertiary acid to have a carbonyl group attached. Primary Halogenoalkanes SN2 Mechanism • Substitution nucleophilic bimolecular • Bimolecular: depends on the concentration of both reactants (halogenoalkane and –OH) Tertiary Halogenoalkanes SN1 Mechanism • Substitution nucleophilic unimolecular • Depends only on the concentration of the halogenoalkane • Steric hindrance caused by three alkyl groups around carbon – Bulky groups makes it difficult for an incoming group to attack the carbon atom Tertiary Halogenoalkanes SN1 Mechanism • Both reactions involve heterolytic fission of C-Cl bond Secondary Halogenoalkanes mechanisms • Secondary halogenoalkanes undergo a mixtures of SN1 and SN2 reactions, depending on reaction conditions M&D questions • The structures of morphine and diamorphine (heroin) are shown in Table 20 of the Data Booklet. State the name of a functional group present in diamorphine (heroin) but not in morphine. State the name of the functional group circled on the structure of caffeine. ........................................................................................................................... (ii) Deduce which functional group is common to both nicotine and caffeine State the differences between the structures of morphine and diamorphine (heroin). State the names of all functional groups in the molecule of morphine. • Examples of strong analgesics are morphine, codeine and diamorphine (heroin). • Their structures are shown in Table 20 of the Data Booklet. • (i) Identify two functional groups present in all three of these analgesics. • (ii)Identify one functional group present in morphine, but not in diamorphine. Identify the amine functional group in the morphine molecule below by drawing a ring around it. (ii) Classify the type of amine present in morphine. ............................................................................................................................. ......... (iii) State the name of the functional group found in heroin but not in morphine. ...................................................................................................................................... Ampicillin is a semi-synthetic penicillin used to treat lung infections. The structure of the antibiotic is shown below. Identify two functional groups present in the side chain (R) of ampicillin by comparing its structure to that of penicillin in Table 20 in the Data Booklet. Caffeine is a stimulant with the following structure. O CH3 CH3 N N O N N CH3 Caffeine (a) Determine whether both amine groups in caffeine are primary, secondary or tertiary. ............................................................................................................................. ........ (b) Caffeine contains the group O CH 3 C N . State the general name for this functional group. ............................................................................................................................. ........ Tablets of the drug Ecstasy are sometimes contaminated with a substance called 4−MTA. H O CH2 N CH H2 C CH3 O CH2 CH3 N CH H3 C CH3 S Ecstasy (i) H 4–MTA Ecstasy and 4-MTA are sympathomimetic drugs. Identify the structural similarity between the two drugs and epinephrine (adrenaline), the structure of which is given in Table 20 of the Data Booklet. H ID ONLY • CH3-O-CH2-CH3 • CH3—CH2—NH2 methoxyethane (ether) ethylamine (amine) • CH3—CH2—C—NH2 Propanamide (amide) O • Objective: SWBAT demonstrate their knowledge of organic chemistry Quiz today Warm up: What is a homologous series?