* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download The Ocean Takes Shape
History of climate change science wikipedia , lookup
Water pollution wikipedia , lookup
History of geomagnetism wikipedia , lookup
Spherical Earth wikipedia , lookup
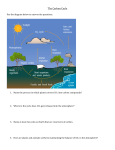
Physical oceanography wikipedia , lookup
History of geology wikipedia , lookup
Age of the Earth wikipedia , lookup
Global Energy and Water Cycle Experiment wikipedia , lookup
Atmosphere of Earth wikipedia , lookup
History of Earth wikipedia , lookup
1 SI Task Instrurction Science Mr. Kaiser Week of April 23 to 27 Main idea: How did oceans form? New York Standard: 1.2f Earth's oceans formed as a result of precipitation over millions of years. The presence of an early ocean is indicated by sedimentary rocks of marine origin, dating back about four billion years. Textbook: Earth Science, Tarbuck/Lutgens 13.1 Precambrian time; 14.1 The Vast Ocean World Schedule Mon: In the textbook, read p.364-368, and 396-398 Tue: Read the packet "How the oceans form?" (attached in this file.) Wed: Students answer questions #1 to 6, in complete, well-written paragraphs. Thur: Students answer questions #7 to 12, in complete, well-written paragraphs. Fri: Homework is due before lunchtime. 2 How did oceans form? Excerpted and adapted from http://science.howstuffworks.com/how-the-ocean-came-to-be-info.htm 4.5 billion years ago: Our solar system formed out of a cosmic cloud of gas and dust. Hydrogen and Helium gas collected to form our Sun. Silicon, oxygen, some regular metals, some radioactive metals, collected to form the inner planets: Mercury, Venus, Earth and Mars. The early Earth was a dry, cratered ball. It had no life, no ocean and no continents. It was a horribly hot and violent place. Many asteroids, comets, and other debris were left over from the solar system's formation. This debris continually bombarded Earth, releasing huge amounts of heat. Radioactive metals inside the earth also kept the Earth very hot. 3 At the same time, frequent volcanic eruptions covered much of the planet's surface in red-hot flows of lava. The early Earth's surface was hot enough to turn any liquid water instantly into steam. Earth eventually obtained enough water to eventually form a vast ocean. How? http://en.wikipedia.org/wiki/Origin_of_water_on_Earth Scientific debate! Comets are mostly made of water ice. We're already pretty sure that comets have delivered a lot of water to Earth, during the millions of years that they pummeled Earth. Thus, some researchers hypothesized that comets brought most of the Earth's water to us. 4 So, our oceans came from comets from outer space? That seems reasonable, as we can see comets still bringing water to Earth (and other planets) even today. But other scientists dispute this! They said that most of the water in our oceans was already here to begin with! In this view, water was in the nebula (huge gaseous cloud) that the Earth was formed out of. Hmm, that seems less likely, because Earth's solid rock doesn't appear to have much water in it. However, chemists have proven that many rocks naturally contain H2O (water). It's just locked in with other molecules, ands in the right circumstances this water can be released. So who is correct? There are ways to find out - and it depends on us knowing that there are actually three different kinds of water - because there are three different kinds of Hydrogen atom! Regular Hydrogen Heavier hydrogen Most heavy hydrogen 1 proton 1 proton 1 proton 1 electron 1 electron 1 electron 0 neutrons 1 neutron 2 neutrons 5 Ordinary water is composed of one oxygen atom and two hydrogen atoms. Heavy-water contains at least one atom of deuterium, a form of hydrogen that has twice the mass of ordinary hydrogen. So who is right? Conduct an experiment! In the 1980's and 1990's, scientists used radio telescopes to analyze the chemical makeup of three comets, as they came close to Earth. This revealed that comets contain 2 times to 20 times more “heavy water” (relative to “normal” water) than is found in the ocean. This much higher proportion of deuterium in comet ice suggests that comets could not have been a major source of our ocean water. The new consensus (what most people agree on) The water in our ocean originated from water molecules in the nebula - the cloud of gas and dust that gave rise to the solar system. As Earth formed, these water molecules became trapped in porous rock, deep inside the hot planet. The water later boiled out as steam. 6 Ok, but how did the water get into a nebula to begin with? Water is made from Hydrogen (H) and oxygen (O). Hydrogen gas has always been around. It was created by the Big Bang, the origin of our universe. Oxygen is naturally created in the interiors of stars, when an older star explodes in a huge explosion called a supernova. (Actually, all elements are made this way.) When the O from a supernova combines with H, it then forms water. For hundreds of millions of years, volcanoes ejected the water vapor along with other hot gases into the Earth's atmosphere. The volcanoes spewed out enough water vapor to wrap the entire planet in a dense blanket. (They also spewed out other gases, see the picture!) 7 Eventually, meteorite bombardments and volcanic eruptions subsided. Earth finally cooled, the water vapor to condensed into clouds, and fall as rain. An almost continuous rain, fed by evaporation and volcanic outgassing, may have drenched the planet for some 10 million years. As the deluge persisted, ponds formed in shallow depressions, then grew into lakes. Eventually, they merged to form an ocean, perhaps around 4 billion years ago. The early sea may have covered the entire planet, to an unknown depth, as rain water collected everywhere on the nearly featureless landscape. Raised continents and deep ocean basins had not yet formed. These came later, after geological processes began altering the landscape. As time went by, Earth's outer shell, or crust, broke into a number of rigid pieces, called tectonic plates, more than 2 billion years ago. (This picture hugely exaggerates the cracking) 8 These plates began to move over partially molten rock. Where two tectonic plates collided, layers of rock rose up, forming mountains and other features high enough to be above the water. 9 At sites where plates moved apart, gaps were created that spread farther and farther apart. Magma (liquefied rock) oozed up through cracks at these sites. It hardened to form new floors on the bottom - the ocean floor. As water filled these basins, the planet looked more like the world of today. Plate tectonics continued to change the shapes of the ocean basins and shift the positions of continents. About 360 million years ago, tectonic plates carried a number of land masses together to form a single continent, called Pangaea. About 200 million years ago, Pangaea broke apart into the northern continent of Laurasia and the southern continent of Gondwanaland. The modern basins of the Pacific, Atlantic, and Indian oceans began to take shape about 120 million years ago, when Laurasia broke apart into Eurasia and North America, while Gondwanaland split into Africa, Antarctica, Australia, India, and South America. By 60 million years ago, the global ocean closely resembled the seas of today. 10 Name: ______________________________ Science: How did the oceans form? 1. What was the surface of the Earth originally like, 4.5 billion years ago? (page 2) 2. Why was the early Earth so very hot? 3. Why did some scientists believe that most of Earth's water came here from comets? (pages 3 and 4) 4. How many different types of Hydrogen are there? 5. What is the difference between normal water and heavy water? 11 6. How did we learn that most of our ocean's water probably did not come from comets? 7. So where did most of Earth's water originally come from? 8. Ok, but how did the water get into a nebula to begin with? (page 6) 9. How did this water get out of the solid rocks in the Earth, and into our atmosphere? 10. What are tectonic plates? 12 11. Originally, all land was probably under water. How did land eventually rise high enough up above the water? (page 8) 12. At sites where plates moved apart, gaps were created - how did this form the ocean floor? 13 You don't need to give this section to the students. It is a list of common misconceptions that people have about this subject. It may, however, be interesting reading. Southern Nevada Regional Professional Development Program TIPS: Targeted Interventions for Proficiency in Science http://www.rpdp.net/sciencetips_v2/E12A2.html Common misconceptions associated with this benchmark: 1. Students incorrectly believe Earth’s atmosphere has always been a constant composition or that changes to Earth’s atmosphere occurred only during prehistoric times. Students may have been instructed previously that a specific compositional percentage of nitrogen and oxygen exist in the Earth’s atmosphere and that this percentage has not varied from the earliest to the most recent point of their education. This may lead students to incorrectly believe that the composition of the atmosphere is unchanging. Students may also understand Earth of the past was up to three times hotter than it is today. They grasp the concept of early Earth as a much more geologically dynamic planet then than now and most have learned how the original atmosphere formed during outgassing as countless volcanoes erupted across Earth’s surface. But, their Earth seems a much less active place, with volcanic activity relatively infrequent. If the atmosphere is to change at all, they may incorrectly believe it will be far in the future as Earth cools even more. A research article discussing student misconceptions about weather, including student misconceptions of atmospheric composition is found at http://www.csulb.edu/~lhenriqu/NARST2000.htm. 2. Students incorrectly think the Earth’s atmosphere is a reservoir so vast that it cannot be significantly altered by natural events or by human industry. Students correctly visualize the atmosphere as enveloping the Earth and extending hundreds of kilometers towards space. It seems reasonable to them that pollution from an automobile would no more alter the atmospheric composition than a 1 pound container of table salt would affect an ocean’s salinity. Students have observed first hand the exhaust from a car, or smoke from a stack, simply dissipating throughout the air with no noticeable effects. Even in cities with early morning smog, they will see as the day warms and the winds blow, the smog will disappear, its components distributed across the county, the state, and eventually across the world. An excellent site to learn about human impact on the atmosphere is found at http://www.carbonfootprint.com. 14 3. Students incorrectly believe Earth’s atmosphere is of a strictly terrestrial origin. Students have generally been taught that a little under 5 billion years ago, volcanic outgassing began to create our atmosphere and subsequently filled our oceans. It is their belief that all the gases in Earth’s atmosphere were originally trapped within magma, and have been released over time, first rapidly and then now more slowly. The students do not acknowledge evidence that our atmosphere has been added to by influx of cometary gases contributed as comets burn up in the atmosphere. To learn more about comets and the evolution of Earth’s atmosphere, go to http://smallcomets.physics.uiowa.edu/faq.htmlx 4. Students confuse the enhanced greenhouse effect with issues regarding the ozone hole. The enhanced greenhouse effect and ozone hole are often discussed simultaneously, and therefore, students incorrectly think that these two result in global climate change. In fact, the two phenomena are not closely related. The enhanced greenhouse effect is predicted to result from increased emission of CO 2 and other greenhouse gases due to human activity. This enhanced greenhouse effect would increase global surface temperatures resulting in global climate change. On the other hand, stratospheric O3 depletion does not result in significant increases in global temperatures and climate change. Although ozone holes allow more ultraviolet light from the Sun to reach the Earth’s surface, the amount of the Sun’s energy in this frequency is so much smaller than that received in visible and infrared. To learn more about this misconception, go to http://www.gcrio.org/gwcc/misconceptions.html.