1_Quantum theory_ introduction and principles
... be described in a new theory: the quantum theory (QT). A wave, called wavefunction (r,t), is associated to each object. The well-defined trajectory of an object in CP (the location, r, and momenta, p = m.v, are precisely known at each instant t) is replaced by (r,t) indicating that the particle ...
... be described in a new theory: the quantum theory (QT). A wave, called wavefunction (r,t), is associated to each object. The well-defined trajectory of an object in CP (the location, r, and momenta, p = m.v, are precisely known at each instant t) is replaced by (r,t) indicating that the particle ...
AP Chemistry
... What is the energy in joules of a mole of photons associated with visible light of wavelength 550 nm? ...
... What is the energy in joules of a mole of photons associated with visible light of wavelength 550 nm? ...
BatelaanUpdate
... Charged particles influenced by electromagnetic fields, even when the two never touch? Surely, it can only be quantum physics. But surprisingly, the quantum nature of this particular effect has been disputed. In the phenomenon known as the Aharonov-Bohm effect, magnetic forces seem to act on charged ...
... Charged particles influenced by electromagnetic fields, even when the two never touch? Surely, it can only be quantum physics. But surprisingly, the quantum nature of this particular effect has been disputed. In the phenomenon known as the Aharonov-Bohm effect, magnetic forces seem to act on charged ...
50 POINTS - University at Albany
... (b.) Product of delta-x and delta-p must be greater than or equal to h-bar/2, where delta-p is equal to (1.15 * 6.626e-25) kg*m/s minus (6.626e-25 / 1.15) kg*m/s, using part (a.) (Also is acceptable if doing / and * 0.85. Very similar results). Δx <= h-bar / (2*Δp) = [h / (2 * pi)] / (2 * Δp) = 6.62 ...
... (b.) Product of delta-x and delta-p must be greater than or equal to h-bar/2, where delta-p is equal to (1.15 * 6.626e-25) kg*m/s minus (6.626e-25 / 1.15) kg*m/s, using part (a.) (Also is acceptable if doing / and * 0.85. Very similar results). Δx <= h-bar / (2*Δp) = [h / (2 * pi)] / (2 * Δp) = 6.62 ...
History and the State-of-art in Quantum Computation
... History and the State-of-art in Quantum Computation ...
... History and the State-of-art in Quantum Computation ...
Chapter 38: Quantization
... Einstein’s Postulates Einstein framed three postulates about light quanta and their interaction with matter: 1. Light of frequency f consists of discrete quanta, each of energy E = hf, where h is Planck’s constant h = 6.63 × 10−34 J s. Each photon travels at the speed of light c = 3.00 × 108 m/s. 2 ...
... Einstein’s Postulates Einstein framed three postulates about light quanta and their interaction with matter: 1. Light of frequency f consists of discrete quanta, each of energy E = hf, where h is Planck’s constant h = 6.63 × 10−34 J s. Each photon travels at the speed of light c = 3.00 × 108 m/s. 2 ...
Document
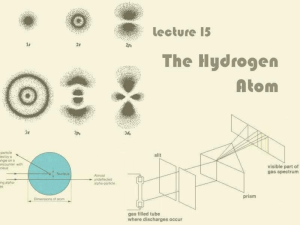
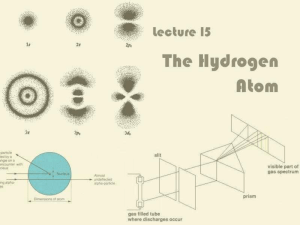
... Light is absorbed by an atom when the electron transition is from lower n to higher n (nf > ni). In this case, DE will be positive. Light is emitted from an atom when the electron transition is from higher n to lower n (nf < ni). In this case, DE will be negative. An electron is ejected when nf = ∞ ...
... Light is absorbed by an atom when the electron transition is from lower n to higher n (nf > ni). In this case, DE will be positive. Light is emitted from an atom when the electron transition is from higher n to lower n (nf < ni). In this case, DE will be negative. An electron is ejected when nf = ∞ ...
photoelectric-effect-qrg
... radiation and independent of the intensity of the radiation. When the photocathode is irradiated with monochromatic light, it is possible to determine Planck’s constant. In addition to confirming Planck’s calculations for the radiation of a black body, Einstein’s interpretation of these experiments ...
... radiation and independent of the intensity of the radiation. When the photocathode is irradiated with monochromatic light, it is possible to determine Planck’s constant. In addition to confirming Planck’s calculations for the radiation of a black body, Einstein’s interpretation of these experiments ...
22-2 Electromagnetic Waves and the Electromagnetic
... Our eyes are sensitive to electromagnetic (EM) waves that have wavelengths in the visible spectrum, between 400 and 700 nm, but our bodies can be affected by EM waves in other ways, too. We have some sensors on the backs of our hands, in particular, that are sensitive to infrared radiation, which we ...
... Our eyes are sensitive to electromagnetic (EM) waves that have wavelengths in the visible spectrum, between 400 and 700 nm, but our bodies can be affected by EM waves in other ways, too. We have some sensors on the backs of our hands, in particular, that are sensitive to infrared radiation, which we ...
Chapter 4 - Tolland High School
... • The Bohr model was more accurate than previous models but was only completely accurate for Hydrogen, other elements did not behave exactly as Bohr predicted • The Quantum model was later developed based on work of many scientists including Schrodinger, Heisenberg, & Einstein ...
... • The Bohr model was more accurate than previous models but was only completely accurate for Hydrogen, other elements did not behave exactly as Bohr predicted • The Quantum model was later developed based on work of many scientists including Schrodinger, Heisenberg, & Einstein ...
Chapter 3 Electromagnetic Theory, Photons, and Light
... In quantum physics for indistinguishable particles: * Bose-Einstein statistics for bosons (particles with integer spin) * Fermi-Dirac statistics for fermions (particles with integer+half spins) Photons are bosons - many photons can simultaneously be in exactly the same state, i.e. have the same ener ...
... In quantum physics for indistinguishable particles: * Bose-Einstein statistics for bosons (particles with integer spin) * Fermi-Dirac statistics for fermions (particles with integer+half spins) Photons are bosons - many photons can simultaneously be in exactly the same state, i.e. have the same ener ...
Ch. 5 PPT Part 2
... of the atom. • Explain the impact of de Broglie's wave article duality and the Heisenberg uncertainty principle on the current view of electrons in atoms. • Identify the relationships among a hydrogen atom's energy levels, sublevels, and atomic orbitals. ...
... of the atom. • Explain the impact of de Broglie's wave article duality and the Heisenberg uncertainty principle on the current view of electrons in atoms. • Identify the relationships among a hydrogen atom's energy levels, sublevels, and atomic orbitals. ...
Ch. 4-2 PowerPoint
... behave as both a particle and a wave. What about electrons? Louis De Broglie stated that electrons could be considered waves confined to a space around an atomic nucleus. ...
... behave as both a particle and a wave. What about electrons? Louis De Broglie stated that electrons could be considered waves confined to a space around an atomic nucleus. ...
How electrons produce color
... have to gain or lose energy to do so. • Each time they lose energy, they emit a bundle of energy. • We see that bundle as a photon! ...
... have to gain or lose energy to do so. • Each time they lose energy, they emit a bundle of energy. • We see that bundle as a photon! ...
Student - Davison Chemistry Website
... 1. Improved Rutherford’s work by saying electrons do not lose energy in the atoms so they will stay in orbit. 2. Stated there are definite levels in which the electrons follow set paths without gaining or losing energy (Planetary Model). 3. Each level has a certain amount of energy associated with i ...
... 1. Improved Rutherford’s work by saying electrons do not lose energy in the atoms so they will stay in orbit. 2. Stated there are definite levels in which the electrons follow set paths without gaining or losing energy (Planetary Model). 3. Each level has a certain amount of energy associated with i ...
The Quantum mechanical model of the atom
... position and momentum of a particle with precision – Heisenberg Uncertainty Principle. Hence, we can picture the electrons like a cloud around the nucleus. Higher density of the cloud = higher probability of finding an electron in that location. ...
... position and momentum of a particle with precision – Heisenberg Uncertainty Principle. Hence, we can picture the electrons like a cloud around the nucleus. Higher density of the cloud = higher probability of finding an electron in that location. ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 2. Prove explicitly that the momentum operator is a self-adjoint operator. 3. Write down the ground state energy eigenfunction of a simple harmonic oscillator? Sketch its graph. 4. Define the parity operator by its effect on a wave function. What are its eigenvalues? 5. If A is any Hermitian operato ...
... 2. Prove explicitly that the momentum operator is a self-adjoint operator. 3. Write down the ground state energy eigenfunction of a simple harmonic oscillator? Sketch its graph. 4. Define the parity operator by its effect on a wave function. What are its eigenvalues? 5. If A is any Hermitian operato ...
History of Atomic theory
... D. He used spectral emission lines to propose a theory that stated that electrons travel in orbits that correspond to specific energy levels. E. Used wave equations to determine the energy states of matter. His theories led to the development of the secondary quantum number. F. He determined that pa ...
... D. He used spectral emission lines to propose a theory that stated that electrons travel in orbits that correspond to specific energy levels. E. Used wave equations to determine the energy states of matter. His theories led to the development of the secondary quantum number. F. He determined that pa ...