TED
... The Quantum Liar Paradox – One atom is found to be excited, which seems to indicate that it emitted no photon. – Hence, it could not interact with the other atom and should not be entangled with it. – But, by violating Bell’s inequality, its “having preserved its photon” is due to entanglement with ...
... The Quantum Liar Paradox – One atom is found to be excited, which seems to indicate that it emitted no photon. – Hence, it could not interact with the other atom and should not be entangled with it. – But, by violating Bell’s inequality, its “having preserved its photon” is due to entanglement with ...
Problem Set 05
... rotational energies of the molecule. [Result: n 2 2 /md 2 ] b) The wavelength of the photon needed to excite the molecule from the n to the n+1 rotational state. [Result: λ=2πcmd 2/(2n+1)] c) The wavelength of the photon need to excite € a nitrogen molecule (N2) from its ground state (n=0) to the ...
... rotational energies of the molecule. [Result: n 2 2 /md 2 ] b) The wavelength of the photon needed to excite the molecule from the n to the n+1 rotational state. [Result: λ=2πcmd 2/(2n+1)] c) The wavelength of the photon need to excite € a nitrogen molecule (N2) from its ground state (n=0) to the ...
Models of the Atom:
... Atoms of the same element are identical. Atoms of different elements are not the same. Atoms of different elements can physically mix together or can chemically combine with one another in simple wholenumber ratios to form COMPOUNDS. ...
... Atoms of the same element are identical. Atoms of different elements are not the same. Atoms of different elements can physically mix together or can chemically combine with one another in simple wholenumber ratios to form COMPOUNDS. ...
Chapter 24 Notes - Valdosta State University
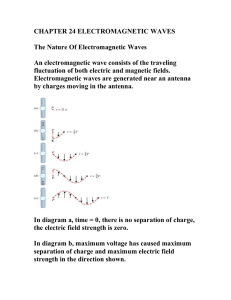
... internal objects. Gamma rays are higher frequency and energy than Xrays. They are produced in nuclear reactions and can cause serious damage to living tissue. Electromagnetic radiation obeys the same relationship between frequency and wavelength as other waves. The equation relating them is: ...
... internal objects. Gamma rays are higher frequency and energy than Xrays. They are produced in nuclear reactions and can cause serious damage to living tissue. Electromagnetic radiation obeys the same relationship between frequency and wavelength as other waves. The equation relating them is: ...
Chapter 6
... corresponding energies. •Each orbital describes a spatial distribution of electron density. •An orbital is described by a set of three quantum numbers. ...
... corresponding energies. •Each orbital describes a spatial distribution of electron density. •An orbital is described by a set of three quantum numbers. ...
Photoelectric Effect
... 1a) List three properties of waves. Examples: 1) Interfere with other waves as they pass through each other. 2) Diffract around objects. 3) Can cause resonance. 4) Can create standing waves 5) Reflect/Refract ...
... 1a) List three properties of waves. Examples: 1) Interfere with other waves as they pass through each other. 2) Diffract around objects. 3) Can cause resonance. 4) Can create standing waves 5) Reflect/Refract ...
schoa - Schieck
... 17. Compare atomic spectra to the continuous spectrum of light. 18. Why are atomic spectra compared to fingerprints? 19. Explain what is meant by the phrase “the energy of electrons in atoms in quantized”. 20. What successes did Bohr’s model of the atom have? 21. What failures did Bohr’s model have? ...
... 17. Compare atomic spectra to the continuous spectrum of light. 18. Why are atomic spectra compared to fingerprints? 19. Explain what is meant by the phrase “the energy of electrons in atoms in quantized”. 20. What successes did Bohr’s model of the atom have? 21. What failures did Bohr’s model have? ...
Electromagnetic Theory, Photons and Light • Introduction – Maxwell
... suggested that electromagnetic waves might be radically different from other waves - perhaps local concentrations of radiant energy existed. ...
... suggested that electromagnetic waves might be radically different from other waves - perhaps local concentrations of radiant energy existed. ...
Light problems
... b. wavelength of electromagnetic energy gained by an atom. c. minimum quantity of energy that can be lost or gained by an atom. d. continuous spectrum of energy given off by an atom. 9.____ A form of energy that exhibits wave behavior as it travels through space is a. microwave radiation. b. ultravi ...
... b. wavelength of electromagnetic energy gained by an atom. c. minimum quantity of energy that can be lost or gained by an atom. d. continuous spectrum of energy given off by an atom. 9.____ A form of energy that exhibits wave behavior as it travels through space is a. microwave radiation. b. ultravi ...
Problem-set-6
... state is at E=0 and the ionization energy has a positive value. Treat Sodium (Natrium) further as a oneelectron system (Z=1), with a single electron outside a closed shell. a) Calculate the Rydberg constant RNa for the Sodium atom (mass =23). (Hint: reduced mass ...
... state is at E=0 and the ionization energy has a positive value. Treat Sodium (Natrium) further as a oneelectron system (Z=1), with a single electron outside a closed shell. a) Calculate the Rydberg constant RNa for the Sodium atom (mass =23). (Hint: reduced mass ...
Quantum Physics Cumulative Review
... b) Give an example of each phenomenon. 5. All lasers bear warnings to avoid shining them into anyone’s eyes. This is because the bright light produced by a laser might cause damage to the back of someone’s eye. Why is a laser’s light bright enough to be dangerous, even when they run at lower power t ...
... b) Give an example of each phenomenon. 5. All lasers bear warnings to avoid shining them into anyone’s eyes. This is because the bright light produced by a laser might cause damage to the back of someone’s eye. Why is a laser’s light bright enough to be dangerous, even when they run at lower power t ...
Quantum Numbers Primer The quantum numbers
... Consider the hydrogen atom. When the electron occupies the n = 1 orbital (the lowest value allowed), the atom is at the lowest energy and the radius is the smallest. We call this lowest energy state the ground state. We can promote an electron in the n = 1 energy level to a higher level; such as n = ...
... Consider the hydrogen atom. When the electron occupies the n = 1 orbital (the lowest value allowed), the atom is at the lowest energy and the radius is the smallest. We call this lowest energy state the ground state. We can promote an electron in the n = 1 energy level to a higher level; such as n = ...
x 100 QUANTUM NUMBERS AND SYMBOLS
... number is 3. What are the possible value sets (l, ml, ms) for the other three quantum numbers? 2. Is it possible for an electron in an atom to have these quantum numbers: n=2, l =1, ml =3, ms =1/2? Why or why not? ...
... number is 3. What are the possible value sets (l, ml, ms) for the other three quantum numbers? 2. Is it possible for an electron in an atom to have these quantum numbers: n=2, l =1, ml =3, ms =1/2? Why or why not? ...
Widener University Summer 2004 ENVR 261 Modern Physics Name
... Explain how the energy bands of metals, semiconductors, and insulators account for the following general optical properties: (a) Metals are opaque to visible light, (b) Many insulators, such as diamond, are transparent to visible light, and (c) semiconductors are opaque to visible light, but transpa ...
... Explain how the energy bands of metals, semiconductors, and insulators account for the following general optical properties: (a) Metals are opaque to visible light, (b) Many insulators, such as diamond, are transparent to visible light, and (c) semiconductors are opaque to visible light, but transpa ...
ONE-ELECTRON ATOMS: SPECTRAL PATTERNS Late 19th
... mass particles, like electrons. In other words, both light and matter can exhibit particle-like and wave-like behavior. If so, he argues, electrons (and perhaps other, very light particles) ought to have wave-like properties: they won’t be localized, but exist over some spatial extent; they will hav ...
... mass particles, like electrons. In other words, both light and matter can exhibit particle-like and wave-like behavior. If so, he argues, electrons (and perhaps other, very light particles) ought to have wave-like properties: they won’t be localized, but exist over some spatial extent; they will hav ...
The Trouble with Gravity Summary/Review
... – According to Newton, gravity affects anything with mass. But according to Einstein, mass is just one form of energy. It follows that gravity actually affects any form of energy. – Because of this, it is the only one of the fundamental forces that notices what the total energy of a system is, rathe ...
... – According to Newton, gravity affects anything with mass. But according to Einstein, mass is just one form of energy. It follows that gravity actually affects any form of energy. – Because of this, it is the only one of the fundamental forces that notices what the total energy of a system is, rathe ...
Welcome to Physics 112N
... • Complimentary’ properties and uncertainty • Mutual Exclusiveness – One quantity being measured precludes measurement of the other ...
... • Complimentary’ properties and uncertainty • Mutual Exclusiveness – One quantity being measured precludes measurement of the other ...