Electrons BellwoodNotes
... The kind of light given off (emitted) by each element is UNIQUE because each element has a UNIQUE arrangement of electrons! (Think fingerprint) (It’s not just about electrons in “shells”… it’s more complicated than that! ...
... The kind of light given off (emitted) by each element is UNIQUE because each element has a UNIQUE arrangement of electrons! (Think fingerprint) (It’s not just about electrons in “shells”… it’s more complicated than that! ...
Lecture 18 (Slides) October 4
... Schrodinger equation, can be factored into an angular and a radial part if we employ spherical polar coordinates. The use of these coordinates makes it especially easy to locate nodes (regions of zero “electron density”) and to represent 3 dimensional probabilities (i.e. represent in 3 dimensions th ...
... Schrodinger equation, can be factored into an angular and a radial part if we employ spherical polar coordinates. The use of these coordinates makes it especially easy to locate nodes (regions of zero “electron density”) and to represent 3 dimensional probabilities (i.e. represent in 3 dimensions th ...
Foundations of Classical and Quantum Electrodynamics Brochure
... 4.2 The Motion of Charged Particles in Electromagnetic Fields. Transformation of the Electric Field 280 4.2.1 Interaction of Charged Particles with the Electromagnetic Field 280 4.2.2 Equations of Motion of a Relativistic Particle 282 4.2.3 Transformations of Electromagnetic Field Stress 288 4.2.4 D ...
... 4.2 The Motion of Charged Particles in Electromagnetic Fields. Transformation of the Electric Field 280 4.2.1 Interaction of Charged Particles with the Electromagnetic Field 280 4.2.2 Equations of Motion of a Relativistic Particle 282 4.2.3 Transformations of Electromagnetic Field Stress 288 4.2.4 D ...
Copyright © 2014 Edmentum - All rights reserved. AP Physics
... I. There is an inherent indeterminancy in the position and momentum of particles. II. The energy of atomic oscillations occurs in exact multiples of a discrete unit. III. Electrons, atoms, and all particles with momentum also exist as waves. IV. No two electrons in an atom may have the same set of q ...
... I. There is an inherent indeterminancy in the position and momentum of particles. II. The energy of atomic oscillations occurs in exact multiples of a discrete unit. III. Electrons, atoms, and all particles with momentum also exist as waves. IV. No two electrons in an atom may have the same set of q ...
Jan. 23, 2006
... from excited-state atoms emitting a photon and falling down to a lower quantum-number ...
... from excited-state atoms emitting a photon and falling down to a lower quantum-number ...
quantumwaves
... •Analogy: Electromagnetic Waves •Described by electric and magnetic fields •What is an electric field? •It is defined in terms of a potential for something American Heritage Science Dictionary “The distribution in space of the strength and direction of forces that would be exerted on an electric cha ...
... •Analogy: Electromagnetic Waves •Described by electric and magnetic fields •What is an electric field? •It is defined in terms of a potential for something American Heritage Science Dictionary “The distribution in space of the strength and direction of forces that would be exerted on an electric cha ...
Electro-magnetic radiation (light)
... Light is emiaed when atoms vibrate (or oscillate), but they can only oscillate with an energy given by: • E = nhν ...
... Light is emiaed when atoms vibrate (or oscillate), but they can only oscillate with an energy given by: • E = nhν ...
Paper - Kendriya Vidyalaya IIT Chennai
... (ii) Light emerges out of a convex lens, when a point source is placed at its focus (c) State Huygens principle. With the suitable diagram, prove law of reflection using Huygens principle. 29. (a)Draw a circuit diagram of a common emitter amplifier using p-n-p transistor. Show input and output volta ...
... (ii) Light emerges out of a convex lens, when a point source is placed at its focus (c) State Huygens principle. With the suitable diagram, prove law of reflection using Huygens principle. 29. (a)Draw a circuit diagram of a common emitter amplifier using p-n-p transistor. Show input and output volta ...
Chapter 8 The quantum theory of motion
... Classical mechanics It is impossible for a particle to surmount over a barrier with potential energy high than its kinetic energy. Quantum mechanics If the barrier is thin and the barrier energy is not infinite, particles have the probability to penetrate into the potential region forbidden by class ...
... Classical mechanics It is impossible for a particle to surmount over a barrier with potential energy high than its kinetic energy. Quantum mechanics If the barrier is thin and the barrier energy is not infinite, particles have the probability to penetrate into the potential region forbidden by class ...
poster
... itself, and then collapses to a point upon detection. Copenhagen/Agnostic (C/A): Electrons are modeled in terms of waves or particles accordingly; emphasis on mathematical calculation (predicting features of the interference pattern) over interpretation. ...
... itself, and then collapses to a point upon detection. Copenhagen/Agnostic (C/A): Electrons are modeled in terms of waves or particles accordingly; emphasis on mathematical calculation (predicting features of the interference pattern) over interpretation. ...
Quantum Chemical Simulations and Descriptors
... The main objective of density functional theory is to replace the many-body electronic wave function with the electronic density as the basic quantity. Whereas the many-body wavefunction is dependent on 3N variables, three spatial variables for each of the N electrons, the density is only a function ...
... The main objective of density functional theory is to replace the many-body electronic wave function with the electronic density as the basic quantity. Whereas the many-body wavefunction is dependent on 3N variables, three spatial variables for each of the N electrons, the density is only a function ...
Problem Set 12
... Problem 1: Consider the Pauli-Schrödinger equation in three space dimensions discussed in class. • Find all the solutions to this equation, and show how the momentum operator and space-translations act on solutions. • Show explicitly how the double-cover of the Euclidean group acts on the space of ...
... Problem 1: Consider the Pauli-Schrödinger equation in three space dimensions discussed in class. • Find all the solutions to this equation, and show how the momentum operator and space-translations act on solutions. • Show explicitly how the double-cover of the Euclidean group acts on the space of ...
Chemistry CPA Activity Sheet Week of November 18, 2013 Unit
... What scientific contributions and discoveries led to an understanding of the nature of the atom? How are subatomic particles arranged in atoms? How do electron configurations relate to position on the periodic table? What is the nature of matter? How do energy and matter interact? How can we use mod ...
... What scientific contributions and discoveries led to an understanding of the nature of the atom? How are subatomic particles arranged in atoms? How do electron configurations relate to position on the periodic table? What is the nature of matter? How do energy and matter interact? How can we use mod ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 1. Find the determinant value of the Loerntz transformation matrix. 2. State the relation between relativistic energy and relativistic momentum. 3. Define 4-current and write down the continuity equation in terms of it. 4. State the covariant form of Lorentz force equation. 5. Define differential sc ...
... 1. Find the determinant value of the Loerntz transformation matrix. 2. State the relation between relativistic energy and relativistic momentum. 3. Define 4-current and write down the continuity equation in terms of it. 4. State the covariant form of Lorentz force equation. 5. Define differential sc ...
B E , 2012
... b) Derive an expression for the intensity at a point in the region of interference due to superposition of two sinusoidal waves with nearly equal amplitudes. Show graphically the intensity ...
... b) Derive an expression for the intensity at a point in the region of interference due to superposition of two sinusoidal waves with nearly equal amplitudes. Show graphically the intensity ...
doc
... radiation and independent of the intensity of the radiation. When the photocathode is irradiated with monochromatic light, it is possible to determine Planck’s constant. In addition to confirming Planck’s calculations for the radiation of a black body, Einstein’s interpretation of these experiments ...
... radiation and independent of the intensity of the radiation. When the photocathode is irradiated with monochromatic light, it is possible to determine Planck’s constant. In addition to confirming Planck’s calculations for the radiation of a black body, Einstein’s interpretation of these experiments ...
particle in a box the uncertainty principle
... principle are fundamental to nature, and are due to the wave properties of matter. This follows cleanly and logically from the mathematics of waves. As humans, we are left with nagging doubts about the uncertainty principle. How dare nature tell us there are things we cannot know! Surely this is jus ...
... principle are fundamental to nature, and are due to the wave properties of matter. This follows cleanly and logically from the mathematics of waves. As humans, we are left with nagging doubts about the uncertainty principle. How dare nature tell us there are things we cannot know! Surely this is jus ...
Group and phase velocity
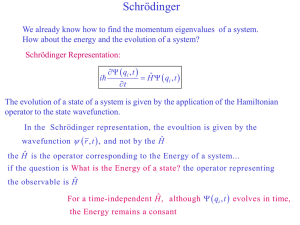
... The evolution of a state of a system is given by the application of the Hamiltonian operator to the state wavefunction. In the Schrodinger representation, the evoultion is given by the wavefunction r , t , and not by the Hˆ ...
... The evolution of a state of a system is given by the application of the Hamiltonian operator to the state wavefunction. In the Schrodinger representation, the evoultion is given by the wavefunction r , t , and not by the Hˆ ...
Lecture 1
... and he had done it in the 17th century. Up until JJ Thomson discovered the electron in the 1890’s, those laws worked for all the particles that people could see. But electrons just didn’t follow F=ma. They could be driven by electrical forces, but their motion was much more jerky than expected in so ...
... and he had done it in the 17th century. Up until JJ Thomson discovered the electron in the 1890’s, those laws worked for all the particles that people could see. But electrons just didn’t follow F=ma. They could be driven by electrical forces, but their motion was much more jerky than expected in so ...