Double-Slit Experiment
... Energy is quantized. It can occur only in discrete units called ______________. de Broglie: Corrected equation to account for relationship between mass and wavelength: m= de Broglie equation: ...
... Energy is quantized. It can occur only in discrete units called ______________. de Broglie: Corrected equation to account for relationship between mass and wavelength: m= de Broglie equation: ...
12/6/16 - Physics
... Note: Some people think of the width fo the wavefunction as being the size of the particle. If so, particles do not have an inherent “size”. They are wave-like and spread out according to their “container” (forces) -- an electron can be microscopic (with uncertain momentum) Or gigantic (with ce ...
... Note: Some people think of the width fo the wavefunction as being the size of the particle. If so, particles do not have an inherent “size”. They are wave-like and spread out according to their “container” (forces) -- an electron can be microscopic (with uncertain momentum) Or gigantic (with ce ...
Part 7 – Quantum physics Useful weblinks Fermilab Inquiring Minds
... This website gives a layman's description of how carbon-14 dating works for objects up to about 50,000 years old. It gives examples and a video describing the dating process for various objects. http://science.howstuffworks.com/environmental/earth/geology/carbon-14.htm Introduction to Atomic Physics ...
... This website gives a layman's description of how carbon-14 dating works for objects up to about 50,000 years old. It gives examples and a video describing the dating process for various objects. http://science.howstuffworks.com/environmental/earth/geology/carbon-14.htm Introduction to Atomic Physics ...
Lecture 9
... This is the Pauli Exclusion Principle An empty orbital is fully described by the three quantum numbers: n, l and ml ...
... This is the Pauli Exclusion Principle An empty orbital is fully described by the three quantum numbers: n, l and ml ...
Exam 1 (Chapters 1-4)
... 2. TV antennas designed to receive electromagnetic radiation from a television broadcasting station may have straight metal rods, a circular loop, or both. Why? 1. The metal rods pick up electric charge from the electromagnetic wave, and the loop provides a conductive path for the current, which cre ...
... 2. TV antennas designed to receive electromagnetic radiation from a television broadcasting station may have straight metal rods, a circular loop, or both. Why? 1. The metal rods pick up electric charge from the electromagnetic wave, and the loop provides a conductive path for the current, which cre ...
Periodic boundary physics etc
... In physics, specifically quantum mechanics, the Schrödinger equation is an equation that describes how the quantum state of a physical system changes in time. It is as central to quantum mechanics as Newton's laws are to classical mechanics. In the standard interpretation of quantum mechanics, the q ...
... In physics, specifically quantum mechanics, the Schrödinger equation is an equation that describes how the quantum state of a physical system changes in time. It is as central to quantum mechanics as Newton's laws are to classical mechanics. In the standard interpretation of quantum mechanics, the q ...
eprint_11_28683_250
... The quantum theory of radiation introduced by Max Planck and Albert Einstein implies a particle theory of light, in addition to the wave theory of light required by the phenomena of interference and diffraction. In 1924, Louis de Broglie argued that if light were composed of particles and yet showed ...
... The quantum theory of radiation introduced by Max Planck and Albert Einstein implies a particle theory of light, in addition to the wave theory of light required by the phenomena of interference and diffraction. In 1924, Louis de Broglie argued that if light were composed of particles and yet showed ...
What`s the Matter?: Quantum Physics for Ordinary People
... Light: Particle or Wave? Albert Einstein explained the photoelectric effect by showing that light energy is quantized. Light, even while exhibiting wave-like interference, comes in particle-like energy packets called photons. What are photons? Certainly not classical particles. When traveling throu ...
... Light: Particle or Wave? Albert Einstein explained the photoelectric effect by showing that light energy is quantized. Light, even while exhibiting wave-like interference, comes in particle-like energy packets called photons. What are photons? Certainly not classical particles. When traveling throu ...
science 1 small-group tutorial scheme
... List all the subshells and orbitals for which the principal quantum number, n, has the values 2 and 3 in order of increasing energy, according to the quantum mechanical rules. Use clearly-labelled diagrams to illustrate the shapes and orientations of the orbitals for which the angular momentum (subs ...
... List all the subshells and orbitals for which the principal quantum number, n, has the values 2 and 3 in order of increasing energy, according to the quantum mechanical rules. Use clearly-labelled diagrams to illustrate the shapes and orientations of the orbitals for which the angular momentum (subs ...
S
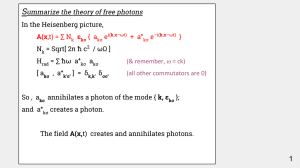
... So , akσ annihilates a photon of the mode { k, εkσ }; and a+kσ creates a photon. The field A(x,t) creates and annihilates photons. ...
... So , akσ annihilates a photon of the mode { k, εkσ }; and a+kσ creates a photon. The field A(x,t) creates and annihilates photons. ...
slides in pdf format
... wave associated with them with wavelength determined by its momentum, λ = h/p. • Bohr’s quantization follows because the electron in an atom is described by a “standing electron wave”. • Experiment: Davisson-Germer (1927) studies electron scattering from crystals - see interference that corresponds ...
... wave associated with them with wavelength determined by its momentum, λ = h/p. • Bohr’s quantization follows because the electron in an atom is described by a “standing electron wave”. • Experiment: Davisson-Germer (1927) studies electron scattering from crystals - see interference that corresponds ...
WAVE MECHANICS (Schrödinger, 1926)
... WAVE MECHANICS * The energy depends only on the principal quantum number, as in the Bohr model: En = -2.179 X 10-18J /n2 * The orbitals are named by giving the n value followed by a letter symbol for l: l= 0,1, 2, 3, 4, 5, ... s p d f g h ... * All orbitals with the same n are called a “shell”. All ...
... WAVE MECHANICS * The energy depends only on the principal quantum number, as in the Bohr model: En = -2.179 X 10-18J /n2 * The orbitals are named by giving the n value followed by a letter symbol for l: l= 0,1, 2, 3, 4, 5, ... s p d f g h ... * All orbitals with the same n are called a “shell”. All ...
CH1710 HW#7 (2017)-Quanta, electron config
... 4. Consider the photon absorbed when a hydrogen electron undergoes a transition from n=1 to n=3. a) Use the Rydberg equation to calculate the wavelength (in Å) of this photon. ...
... 4. Consider the photon absorbed when a hydrogen electron undergoes a transition from n=1 to n=3. a) Use the Rydberg equation to calculate the wavelength (in Å) of this photon. ...
6.007 Lecture 38: Examples of Heisenberg
... Classically we know that negatively charged electron is attracted to the proton, and it was suggested that the electron circles the proton. But if an electron is circling, every-time it changes direction it is accelerated, and an accelerating charge emits EM radiation (light). Classically, it can be ...
... Classically we know that negatively charged electron is attracted to the proton, and it was suggested that the electron circles the proton. But if an electron is circling, every-time it changes direction it is accelerated, and an accelerating charge emits EM radiation (light). Classically, it can be ...
J.
... it exhibits the difficulty of associating the effect of the magnetic field with the sign change of half-integer spin particles under rotations through 2m'. The point is this: Any rotation of a particle with nonzero angular momentum must be effected by applying a torque. Such a torque would be repres ...
... it exhibits the difficulty of associating the effect of the magnetic field with the sign change of half-integer spin particles under rotations through 2m'. The point is this: Any rotation of a particle with nonzero angular momentum must be effected by applying a torque. Such a torque would be repres ...