T1_The_Origins_Of_Quantum_Mechanics
... 1905: …“photons” (g), each of energy E = hf. Think of these now as individual photons instead of a smooth plane wave!! This is at the core of Einstein’s explanation. We now think of light as being made of photons. Each one carries a specific amount of energy, E = hf. It also has other particle prop ...
... 1905: …“photons” (g), each of energy E = hf. Think of these now as individual photons instead of a smooth plane wave!! This is at the core of Einstein’s explanation. We now think of light as being made of photons. Each one carries a specific amount of energy, E = hf. It also has other particle prop ...
L 35 Modern Physics [1]
... Newton’s Laws have flaws! • Newton’s laws, which were so successful in allowing us to understand the behavior of big objects such as the motions of the planets, failed when pushed to explain atomic size phenomena. • The discovery of the laws of atomic physics led to every important 20th century dis ...
... Newton’s Laws have flaws! • Newton’s laws, which were so successful in allowing us to understand the behavior of big objects such as the motions of the planets, failed when pushed to explain atomic size phenomena. • The discovery of the laws of atomic physics led to every important 20th century dis ...
Ch4 notes - Midway ISD
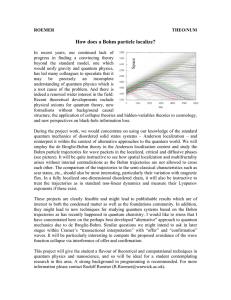
... • Bohr Model of the Atomproposed that hydrogen atom has only certain allowable energy states ...
... • Bohr Model of the Atomproposed that hydrogen atom has only certain allowable energy states ...
Chapter 1: Physics Basics (PDF file)
... If one of the slits is covered up, then the pattern observed on the screen is just the illumination of one slit. If both slits are uncovered, however, the light passing thru the two slits interferes. This interference produces a pattern of several alternating bright and dark lines on the screen and ...
... If one of the slits is covered up, then the pattern observed on the screen is just the illumination of one slit. If both slits are uncovered, however, the light passing thru the two slits interferes. This interference produces a pattern of several alternating bright and dark lines on the screen and ...
Sonication
... In solution, the particles vibrate because as they experience cycles of pressure, microscopic vacuum bubbles form and then collapse into solution, a process called cavitation.1 These vibrations can disrupt molecular interactions (e.g. between molecules of water), break clumps of particles apart, and ...
... In solution, the particles vibrate because as they experience cycles of pressure, microscopic vacuum bubbles form and then collapse into solution, a process called cavitation.1 These vibrations can disrupt molecular interactions (e.g. between molecules of water), break clumps of particles apart, and ...
Chapter 37
... Light from a monochromatic source is used to illuminate a barrier. The barrier contains two small openings. The openings are usually in the shape of slits. The light emerging from the two slits is coherent since a single source produces the original light beam. ...
... Light from a monochromatic source is used to illuminate a barrier. The barrier contains two small openings. The openings are usually in the shape of slits. The light emerging from the two slits is coherent since a single source produces the original light beam. ...
Modern Physics
... from the nucleus for the hydrogen atom is 2 ao. Find the probability of finding the 1-s electron at a distance greater than 2 ao according to quantum mechanics. ...
... from the nucleus for the hydrogen atom is 2 ao. Find the probability of finding the 1-s electron at a distance greater than 2 ao according to quantum mechanics. ...
Quantum Mechanics - UCSD Department of Physics
... • Every particle or system of particles can be defined in quantum mechanical terms – and therefore have wave-like properties ...
... • Every particle or system of particles can be defined in quantum mechanical terms – and therefore have wave-like properties ...
study note 1 06
... Use de Broglie's wave equation to explain why large objects do The huge m means a very small wavelength. not seem like waves Through a diffraction experiment. (showing destructive and constructive How can the wave properties of light be shown? interference) (as in fig. 6.16) Give an example showing ...
... Use de Broglie's wave equation to explain why large objects do The huge m means a very small wavelength. not seem like waves Through a diffraction experiment. (showing destructive and constructive How can the wave properties of light be shown? interference) (as in fig. 6.16) Give an example showing ...
history of the atom ppt student copy
... 4. Atoms of different elements combined in whole-number ratios to form chemical compounds. 5. In chemical reactions, ____________________________________ ________________________________________________________ •Dalton’s theory helped explain the law of conservation of mass because it stated that at ...
... 4. Atoms of different elements combined in whole-number ratios to form chemical compounds. 5. In chemical reactions, ____________________________________ ________________________________________________________ •Dalton’s theory helped explain the law of conservation of mass because it stated that at ...
Class25_review - Rensselaer Polytechnic Institute
... • BUT, can move mirror so no light in bottom! (Color of line is NOT related to actual color of laser; all beams have same wavelength!) ...
... • BUT, can move mirror so no light in bottom! (Color of line is NOT related to actual color of laser; all beams have same wavelength!) ...
9/25 - SMU Physics
... Equal probability to find the particle anywhere the location of this particle is uncertain, although the momentum of this particle is certain. Why? p k ...
... Equal probability to find the particle anywhere the location of this particle is uncertain, although the momentum of this particle is certain. Why? p k ...
Chapter 4
... Rutherford model…It did not answer: Where the e- were located in the space outside the nucleus Why the e- did not crash into the nucleus Why atoms produce spectra at specific wavelengths ...
... Rutherford model…It did not answer: Where the e- were located in the space outside the nucleus Why the e- did not crash into the nucleus Why atoms produce spectra at specific wavelengths ...
Concept of the Gibbsian ensemble
... Transition from classical to quantum statistics In classical mechanics a state of a system is determined by knowledge of position, q, and momentum, p. Dynamic evolution given by : trajectory in -space ...
... Transition from classical to quantum statistics In classical mechanics a state of a system is determined by knowledge of position, q, and momentum, p. Dynamic evolution given by : trajectory in -space ...
e - Leon County Schools
... certain amounts of energy -- e– could be only at certain distances from nucleus planetary (Bohr) model ...
... certain amounts of energy -- e– could be only at certain distances from nucleus planetary (Bohr) model ...
1 PHY4605–Introduction to Quantum Mechanics II Spring 2004 Test 1 Solutions
... (d) Now a camera with a very narrow-angle lens is placed at position C pointing along the −y axis and another ρ in the same state as (c) is injected into the SG region. The camera does not observe the particle during the time it takes for the particle to pass through the setup. What is the probabili ...
... (d) Now a camera with a very narrow-angle lens is placed at position C pointing along the −y axis and another ρ in the same state as (c) is injected into the SG region. The camera does not observe the particle during the time it takes for the particle to pass through the setup. What is the probabili ...


![L 35 Modern Physics [1]](http://s1.studyres.com/store/data/008517000_1-9aef89c0ca089782f518550164188024-300x300.png)