Kinetic Theory
... The gas consists of a large number of identical particles of mass m. The particles have negligible size and no internal ...
... The gas consists of a large number of identical particles of mass m. The particles have negligible size and no internal ...
Lec-23_Strachan
... As a general rule, the order that electrons fill an atom’s subshell is: Once one subshell is filled, the next electron goes into the vacant subshell that is lowest in energy Otherwise, the electron would radiate energy until it reached the subshell with the lowest energy A subshell is filled when it ...
... As a general rule, the order that electrons fill an atom’s subshell is: Once one subshell is filled, the next electron goes into the vacant subshell that is lowest in energy Otherwise, the electron would radiate energy until it reached the subshell with the lowest energy A subshell is filled when it ...
Slides - WFU Physics
... 2. Solve Green’s function equations in curved spacetime S x, x 4 x x ' 3. Use Green’s functions to calculate expectation value of T ...
... 2. Solve Green’s function equations in curved spacetime S x, x 4 x x ' 3. Use Green’s functions to calculate expectation value of T ...
Problem set 7
... (d) Use the previous result to simplify the time-ordered exponential formula for U(t) assuming A and B commute with their commutator. (e) Assuming A and B commute with their commutator, show that ...
... (d) Use the previous result to simplify the time-ordered exponential formula for U(t) assuming A and B commute with their commutator. (e) Assuming A and B commute with their commutator, show that ...
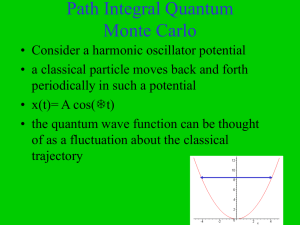
Chapter 2 Waves and Particles De Broglie wavelength: λ=h/p, where
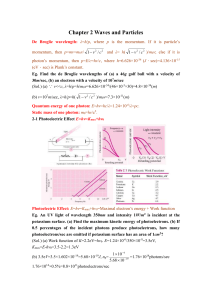
... Eg. An UV light of wavelength 350nm and intensity 1W/m2 is incident at the potassium surface. (a) Find the maximum kinetic energy of photoelectrons. (b) If 0.5 percentages of the incident photons produce photoelectrons, how many photoelectrons/sec are emitted if potassium surface has an area of 1cm2 ...
... Eg. An UV light of wavelength 350nm and intensity 1W/m2 is incident at the potassium surface. (a) Find the maximum kinetic energy of photoelectrons. (b) If 0.5 percentages of the incident photons produce photoelectrons, how many photoelectrons/sec are emitted if potassium surface has an area of 1cm2 ...
Sample pages 1 PDF
... movement of a neutron with the same speed (103 m/s) is associated with a wave of wavelength λ ≈ 4 × 10−13 m. In other words, a neutron moving with that speed can be considered a de Broglie wave with a wavelength of 4 × 10−13 m. Similar wavelengths are characteristic of cosmic rays. Particles with a ...
... movement of a neutron with the same speed (103 m/s) is associated with a wave of wavelength λ ≈ 4 × 10−13 m. In other words, a neutron moving with that speed can be considered a de Broglie wave with a wavelength of 4 × 10−13 m. Similar wavelengths are characteristic of cosmic rays. Particles with a ...
Raman spectroscopy
... a particle of mass m with respect to a chosen origin is given by L = mvr sin θ ...
... a particle of mass m with respect to a chosen origin is given by L = mvr sin θ ...