Effects of Molecular Crowding on Binding Affinity of Dihydrofolate to
... DHFR is not only found in mammals, but also in bacteria. One isoform is the R67 DHFR, which is carried by an R-plasmid or resistance plasmid. In comparison to the chromosomal DHFR, R67 has different characteristics. For instance, R67 DHFR has a lower affinity for DHF than the chromosomal form 1. Ad ...
... DHFR is not only found in mammals, but also in bacteria. One isoform is the R67 DHFR, which is carried by an R-plasmid or resistance plasmid. In comparison to the chromosomal DHFR, R67 has different characteristics. For instance, R67 DHFR has a lower affinity for DHF than the chromosomal form 1. Ad ...
Lecture 2 - cholesterol _CVS block
... Most important animal steroid Mainitains membrane fluidity Has an insulating effect on nerve fibres Cholesterol is the parent molecule for – Bile acids and bile salts – Steroid hormones and – vitamin D3 ...
... Most important animal steroid Mainitains membrane fluidity Has an insulating effect on nerve fibres Cholesterol is the parent molecule for – Bile acids and bile salts – Steroid hormones and – vitamin D3 ...
13-Krebs cycle
... The citric acid cycle – also known as the tricarboxylic acid (TCA) cycle or the Krebs cycle– is a series of chemical reactions used by all aerobic organisms to generate energy through the oxidation of acetyl-CoA derived from carbohydrates, fats and proteins into carbon dioxide and chemical energy in ...
... The citric acid cycle – also known as the tricarboxylic acid (TCA) cycle or the Krebs cycle– is a series of chemical reactions used by all aerobic organisms to generate energy through the oxidation of acetyl-CoA derived from carbohydrates, fats and proteins into carbon dioxide and chemical energy in ...
13-Krebs cycle
... The citric acid cycle – also known as the tricarboxylic acid (TCA) cycle or the Krebs cycle– is a series of chemical reactions used by all aerobic organisms to generate energy through the oxidation of acetyl-CoA derived from carbohydrates, fats and proteins into carbon dioxide and chemical energy in ...
... The citric acid cycle – also known as the tricarboxylic acid (TCA) cycle or the Krebs cycle– is a series of chemical reactions used by all aerobic organisms to generate energy through the oxidation of acetyl-CoA derived from carbohydrates, fats and proteins into carbon dioxide and chemical energy in ...
Non-Essential Amino Acids
... generally depends on our hormones. And herein lays the key to weight loss: the systematic supplementation of certain amino acids allows us to stimulate the body to produce enough fatburning hormones – in a natural manner and in harmony with the body's needs. One important fat-burning hormone is the ...
... generally depends on our hormones. And herein lays the key to weight loss: the systematic supplementation of certain amino acids allows us to stimulate the body to produce enough fatburning hormones – in a natural manner and in harmony with the body's needs. One important fat-burning hormone is the ...
Biochemical Characterization of 2-Nitropropane Dioxygenase from
... nitrite, with oxygen as the electron acceptor. Although nitroalkanes are anticipated to be toxic and carcinogenic, they are used widely in chemical industry for a quick and effective way of synthesizing common reagents. Consequently, the biochemical and biophysical analysis of 2nitropropane dioxygan ...
... nitrite, with oxygen as the electron acceptor. Although nitroalkanes are anticipated to be toxic and carcinogenic, they are used widely in chemical industry for a quick and effective way of synthesizing common reagents. Consequently, the biochemical and biophysical analysis of 2nitropropane dioxygan ...
PFK-2
... • Phosphofructokinase- major control point; first enzyme “unique” to glycolysis • Hexokinase or glucokinase • Pyruvate kinase •Phosphofructokinase responds to changes in: • Energy state of the cell (high ATP levels inhibit) • H+ concentration (high lactate levels inhibit) • Availability of alternate ...
... • Phosphofructokinase- major control point; first enzyme “unique” to glycolysis • Hexokinase or glucokinase • Pyruvate kinase •Phosphofructokinase responds to changes in: • Energy state of the cell (high ATP levels inhibit) • H+ concentration (high lactate levels inhibit) • Availability of alternate ...
Chapter 17
... • Some introns contain sequences that may regulate gene expression • Some genes can encode more than one kind of polypeptide, depending on which segments are treated as exons during splicing • This is called alternative RNA splicing • Consequently, the number of different proteins an organism can pr ...
... • Some introns contain sequences that may regulate gene expression • Some genes can encode more than one kind of polypeptide, depending on which segments are treated as exons during splicing • This is called alternative RNA splicing • Consequently, the number of different proteins an organism can pr ...
Human Physiology An Integrated Approach 6/E
... how combinations of these molecules acquire the remarkable attributes of a living cell? How can living cells carry out processes that far exceed what we would predict from understanding their individual components? The answer is emergent properties , those distinctive traits that cannot be predicted ...
... how combinations of these molecules acquire the remarkable attributes of a living cell? How can living cells carry out processes that far exceed what we would predict from understanding their individual components? The answer is emergent properties , those distinctive traits that cannot be predicted ...
NZY First-Strand cDNA Synthesis Kit
... mRNA copies of the GAPDH gene in mouse liver cells. Precisely, 0.5 µg of total RNA extracted from mouse liver is used as starting template material. ...
... mRNA copies of the GAPDH gene in mouse liver cells. Precisely, 0.5 µg of total RNA extracted from mouse liver is used as starting template material. ...
PRACTICE SET 6 - UC Davis Plant Sciences
... Compare and contrast the pathway by which fatty acids are degraded (betaoxidation) with the pathway by which fatty acids are synthesized from acetate. Cover such points as: (a) nature of the "activated" structures; (b) coenzymes involved; (c) stereochemistry of the intermediates; (d) the places in t ...
... Compare and contrast the pathway by which fatty acids are degraded (betaoxidation) with the pathway by which fatty acids are synthesized from acetate. Cover such points as: (a) nature of the "activated" structures; (b) coenzymes involved; (c) stereochemistry of the intermediates; (d) the places in t ...
ch 17 from gene to protein
... • Some introns contain sequences that may regulate gene expression • Some genes can encode more than one kind of polypeptide, depending on which segments are treated as exons during splicing • This is called alternative RNA splicing • Consequently, the number of different proteins an organism can pr ...
... • Some introns contain sequences that may regulate gene expression • Some genes can encode more than one kind of polypeptide, depending on which segments are treated as exons during splicing • This is called alternative RNA splicing • Consequently, the number of different proteins an organism can pr ...
Chapter 20 Notes
... • CO2 binds weakly to the enzyme, but oxaloacetate binds tightly, so the reaction goes the wrong way. ...
... • CO2 binds weakly to the enzyme, but oxaloacetate binds tightly, so the reaction goes the wrong way. ...
ILA: DIABETES
... • Although the blood glucose level is high but glucose is not taken up by the cells due to insulin deficiency therefore the cells are starved • The patient will take more food (polyphagia) to compensate for the loss of glucose and also loss of protein ...
... • Although the blood glucose level is high but glucose is not taken up by the cells due to insulin deficiency therefore the cells are starved • The patient will take more food (polyphagia) to compensate for the loss of glucose and also loss of protein ...
Nucleotides: Synthesis and Degradation
... – If endergonic reaction released energy into cell as heat energy, wouldn’t be useful – Must be coupled to an exergonic reaction ...
... – If endergonic reaction released energy into cell as heat energy, wouldn’t be useful – Must be coupled to an exergonic reaction ...
6b How to ID an Unk organism
... reagent. If a red ring forms at the top of the tube, it is positive for indole, so the organism makes tryptophan deaminase. Kovac’s reagent has alcohol in it. Alcohol is lighter than water, so when the test is positive and turns red, the red ring floats to the top of the tube. (Control = E. coli). T ...
... reagent. If a red ring forms at the top of the tube, it is positive for indole, so the organism makes tryptophan deaminase. Kovac’s reagent has alcohol in it. Alcohol is lighter than water, so when the test is positive and turns red, the red ring floats to the top of the tube. (Control = E. coli). T ...
a guide-book to biochemistry
... without damage to their catalytic function, and many have been obtained in crystalline form. Already the study of their behaviour has thrown light on what may be called the physical chemistry of life, and pathways of chemical transformation can be followed in detail by allowing purified enzymes in t ...
... without damage to their catalytic function, and many have been obtained in crystalline form. Already the study of their behaviour has thrown light on what may be called the physical chemistry of life, and pathways of chemical transformation can be followed in detail by allowing purified enzymes in t ...
Fall, 2002
... their amine groups (-NH2). The reaction is a dehydration between the amine and the carbonyl group with the formationof a C=N double bond. Write the equation for this reaction with this simple amine, CH3-NH2. ...
... their amine groups (-NH2). The reaction is a dehydration between the amine and the carbonyl group with the formationof a C=N double bond. Write the equation for this reaction with this simple amine, CH3-NH2. ...
Poster
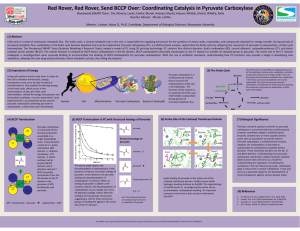
... Cells exist in a state of continuous metabolic flux. The Krebs cycle, a central metabolic hub in the cell, is responsible for supplying precursors for the synthesis of amino acids, nucleotides, and compounds required for energy transfer. During periods of increased metabolic flux, metabolites in the ...
... Cells exist in a state of continuous metabolic flux. The Krebs cycle, a central metabolic hub in the cell, is responsible for supplying precursors for the synthesis of amino acids, nucleotides, and compounds required for energy transfer. During periods of increased metabolic flux, metabolites in the ...
Regulation of Protein Degradation
... vivo. Dramatic differences in the in vivo stability of different proteins have been documented. Multiple proteins have been demonstrated to undergo selective proteolysis only at particular stages in the cell cycle, after certain environmental stimuli, or after specific metabolic or developmental cha ...
... vivo. Dramatic differences in the in vivo stability of different proteins have been documented. Multiple proteins have been demonstrated to undergo selective proteolysis only at particular stages in the cell cycle, after certain environmental stimuli, or after specific metabolic or developmental cha ...
Fuel Metabolism PART 1: Structure and Function of Protein
... GTP (8 kcal). The percentage of the total energy available from oxidation of acetate that is transferred to these compounds is, therefore, 208/243 kcal or 86%. 6-C. About 12 ATP are produced by the TCA cycle (12 x 8 kcal = 96 kcal). The percentage of the total energy available from oxidation of acet ...
... GTP (8 kcal). The percentage of the total energy available from oxidation of acetate that is transferred to these compounds is, therefore, 208/243 kcal or 86%. 6-C. About 12 ATP are produced by the TCA cycle (12 x 8 kcal = 96 kcal). The percentage of the total energy available from oxidation of acet ...
HONORS BIOLOGY MIDTERM EXAM STUDY GUIDE 2016
... 9. Describe the human activities that may be contributing to changes in atmospheric CO2 levels. Describe the effect that increasing CO2 levels may have on the environment. 10. Describe and explain changes that could be made to make the Greenwich High School campus more environmentally friendly. 11. ...
... 9. Describe the human activities that may be contributing to changes in atmospheric CO2 levels. Describe the effect that increasing CO2 levels may have on the environment. 10. Describe and explain changes that could be made to make the Greenwich High School campus more environmentally friendly. 11. ...
Enzyme

Enzymes /ˈɛnzaɪmz/ are macromolecular biological catalysts. Enzymes accelerate, or catalyze, chemical reactions. The molecules at the beginning of the process are called substrates and the enzyme converts these into different molecules, called products. Almost all metabolic processes in the cell need enzymes in order to occur at rates fast enough to sustain life. The set of enzymes made in a cell determines which metabolic pathways occur in that cell. The study of enzymes is called enzymology.Enzymes are known to catalyze more than 5,000 biochemical reaction types. Most enzymes are proteins, although a few are catalytic RNA molecules. Enzymes' specificity comes from their unique three-dimensional structures.Like all catalysts, enzymes increase the rate of a reaction by lowering its activation energy. Some enzymes can make their conversion of substrate to product occur many millions of times faster. An extreme example is orotidine 5'-phosphate decarboxylase, which allows a reaction that would otherwise take millions of years to occur in milliseconds. Chemically, enzymes are like any catalyst and are not consumed in chemical reactions, nor do they alter the equilibrium of a reaction. Enzymes differ from most other catalysts by being much more specific. Enzyme activity can be affected by other molecules: inhibitors are molecules that decrease enzyme activity, and activators are molecules that increase activity. Many drugs and poisons are enzyme inhibitors. An enzyme's activity decreases markedly outside its optimal temperature and pH.Some enzymes are used commercially, for example, in the synthesis of antibiotics. Some household products use enzymes to speed up chemical reactions: enzymes in biological washing powders break down protein, starch or fat stains on clothes, and enzymes in meat tenderizer break down proteins into smaller molecules, making the meat easier to chew.