Biology Organic Molecules Notes
... V. Molecules of Life B.) Proteins 1.) Made of mostly C, H, O, and N 2.) Are long chains of amino acids Joined together by peptide bonds Dipeptide: two amino acids Polypeptide: very long chain of amino acids Proteins all have a different shape but are all globular ...
... V. Molecules of Life B.) Proteins 1.) Made of mostly C, H, O, and N 2.) Are long chains of amino acids Joined together by peptide bonds Dipeptide: two amino acids Polypeptide: very long chain of amino acids Proteins all have a different shape but are all globular ...
Name : ______ Date: ______ per: _____ Physiology II Study guide
... 4. What protein is found in red blood cells where oxygen binds? ________________________________________ 5. Explain where blood goes in the two main pathways: a. Systemic: ___________________________________________________________________________ ...
... 4. What protein is found in red blood cells where oxygen binds? ________________________________________ 5. Explain where blood goes in the two main pathways: a. Systemic: ___________________________________________________________________________ ...
2.2.3 Enzymes
... 2.They speed up chemical reactions inside the cytoplasm. 3.They are needed only in small amounts 4.They remain unchanged after each reaction and can therefore be reused. 5.They are highly specific. (Each enzyme can only work on one chemical reaction. For example, catalase will only work on hydrogen ...
... 2.They speed up chemical reactions inside the cytoplasm. 3.They are needed only in small amounts 4.They remain unchanged after each reaction and can therefore be reused. 5.They are highly specific. (Each enzyme can only work on one chemical reaction. For example, catalase will only work on hydrogen ...
Chemical Basis of Life packet #2-1.answer.key
... 5. A scientist observes that, when the pH of the environment surrounding an enzyme is changed, the rate the enzyme catalyzes a reaction greatly decreases. Which statement best describes how a change in pH can affect an enzyme? A. A pH change can cause the enzyme to change its shape. B. A pH change c ...
... 5. A scientist observes that, when the pH of the environment surrounding an enzyme is changed, the rate the enzyme catalyzes a reaction greatly decreases. Which statement best describes how a change in pH can affect an enzyme? A. A pH change can cause the enzyme to change its shape. B. A pH change c ...
Enzymes lII: Clinical Applications
... substrate concentrations are maintained at saturating levels (i.e., zero-order kinetics with respect to substrate concentration) and other factors (e.g., pH, temperature, and cofactors) are maintained at optimal and constant levels (see Chapter 6). Under these conditions, the rate of substrate remov ...
... substrate concentrations are maintained at saturating levels (i.e., zero-order kinetics with respect to substrate concentration) and other factors (e.g., pH, temperature, and cofactors) are maintained at optimal and constant levels (see Chapter 6). Under these conditions, the rate of substrate remov ...
Basic Chemistry and Biochemistry Unit Review Sheet File
... 11. Measurement of the hydrogen ion concentration of a solution may be given in terms of _________________. 12. Glucose is a __________________________________, maltose is a __________________________, and starch is a _________________________________. 13. The type of reaction by which proteins are ...
... 11. Measurement of the hydrogen ion concentration of a solution may be given in terms of _________________. 12. Glucose is a __________________________________, maltose is a __________________________, and starch is a _________________________________. 13. The type of reaction by which proteins are ...
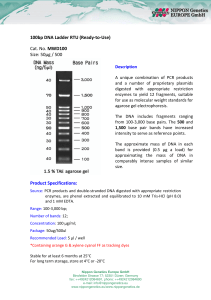
100bp DNA Ladder RTU (Ready-to-Use) Cat. No. MWD100 Size
... 100bp DNA Ladder RTU (Ready-to-Use) Cat. No. MWD100 Size: 50μg / 500 Description A unique combination of PCR products and a number of proprietary plasmids digested with appropriate restriction enzymes to yield 12 fragments, suitable for use as molecular weight standards for agarose gel electrophores ...
... 100bp DNA Ladder RTU (Ready-to-Use) Cat. No. MWD100 Size: 50μg / 500 Description A unique combination of PCR products and a number of proprietary plasmids digested with appropriate restriction enzymes to yield 12 fragments, suitable for use as molecular weight standards for agarose gel electrophores ...
The Clinical Aspects of Enzyme Deficiencies in Haematology
... • Glycogen storage disease Type 1b - Glucose-6-phosphate translocase • Glucose-6-phosphatase catalytic subunit 3 (G6PC3) deficiency • Barth Syndrome – tafazzin (a mitochondrial phopspholipid transacylase) deficiency ...
... • Glycogen storage disease Type 1b - Glucose-6-phosphate translocase • Glucose-6-phosphatase catalytic subunit 3 (G6PC3) deficiency • Barth Syndrome – tafazzin (a mitochondrial phopspholipid transacylase) deficiency ...
Biochemistry-lab-identifying
... Atoms are pure substances like Hydrogen, Oxygen, Carbon, Nitrogen, and Phosphorus. Scientists use the Periodic Table of Elements to organize all pure substances on earth. Elements often join together (or bond) through donating or sharing energy from electrons. When two or more elements join together ...
... Atoms are pure substances like Hydrogen, Oxygen, Carbon, Nitrogen, and Phosphorus. Scientists use the Periodic Table of Elements to organize all pure substances on earth. Elements often join together (or bond) through donating or sharing energy from electrons. When two or more elements join together ...
Ch 2 PowerPoint Notes
... •Even in a chemical reaction that releases energy, activation energy must be supplied before the reaction can occur. Enzymes •Enzymes are substances that increase the speed of chemical reactions. Most enzymes are proteins. •Enzymes are catalysts, which are substances that reduce the activation energ ...
... •Even in a chemical reaction that releases energy, activation energy must be supplied before the reaction can occur. Enzymes •Enzymes are substances that increase the speed of chemical reactions. Most enzymes are proteins. •Enzymes are catalysts, which are substances that reduce the activation energ ...
Enzymes: Introduction
... starting state (S in this case), the "barrier" over which the reaction must go in order to proceed. ∆G‡ has POSITIVE values (∆G‡ > 0) -- it it's s a free energy BARRIER. k is rate constant for the reaction. κ is Boltzmann’s constant and h is Planck’s constant. NOTE: Rate constant k is inversely ...
... starting state (S in this case), the "barrier" over which the reaction must go in order to proceed. ∆G‡ has POSITIVE values (∆G‡ > 0) -- it it's s a free energy BARRIER. k is rate constant for the reaction. κ is Boltzmann’s constant and h is Planck’s constant. NOTE: Rate constant k is inversely ...
Biomolecules Unit Review File
... 12. Draw a single nucleotide. Draw a chain of nucleic acid. How many strands does DNA have? How many strands does RNA have? 13. What provides more energy lipids or carbohydrates? What type of energy are each of them? 14. What is glycogen? Where can you find it? What organisms utilize glycogen? 15. W ...
... 12. Draw a single nucleotide. Draw a chain of nucleic acid. How many strands does DNA have? How many strands does RNA have? 13. What provides more energy lipids or carbohydrates? What type of energy are each of them? 14. What is glycogen? Where can you find it? What organisms utilize glycogen? 15. W ...
6.1 Cellular respiration
... 6.1 Cellular respiration Read pages 66–9 of Human Perspectives Units 1 & 2 and fill in the missing words to complete this summary of cellular respiration. Glucose metabolism Cellular respiration = glucose oxidation Glucose 1 oxygen → carbon dioxide 1 water 1 energy (ATP) This reaction does not occur ...
... 6.1 Cellular respiration Read pages 66–9 of Human Perspectives Units 1 & 2 and fill in the missing words to complete this summary of cellular respiration. Glucose metabolism Cellular respiration = glucose oxidation Glucose 1 oxygen → carbon dioxide 1 water 1 energy (ATP) This reaction does not occur ...
formation of a highly specialized cell type, the spermatozoon. During
... inaccurate histochemical reaction. In addition, biochesolubility mical and histochemical studies have commonly been carried out by use of separate substrates without careful correlation to the composite enzyme pattern with different substrate requirements, possibly present in the cells. The analysis ...
... inaccurate histochemical reaction. In addition, biochesolubility mical and histochemical studies have commonly been carried out by use of separate substrates without careful correlation to the composite enzyme pattern with different substrate requirements, possibly present in the cells. The analysis ...
The FAH Fold Meets the Krebs Cycle
... domain of FAHD1 and other members of the FAH family [9]. This process yielded the prokaryotic enzyme Cg1458 as a promising candidate, previously identified as a soluble ODx [2].Subsequent in vitro analysis of purified recombinant human FAHD1 confirmed that it indeed exhibits ODx activity. Interestin ...
... domain of FAHD1 and other members of the FAH family [9]. This process yielded the prokaryotic enzyme Cg1458 as a promising candidate, previously identified as a soluble ODx [2].Subsequent in vitro analysis of purified recombinant human FAHD1 confirmed that it indeed exhibits ODx activity. Interestin ...
Secondary Structure of Proteins
... Methanol is toxic – 10 mL can cause blindness due to its metabolite formic acid. Treatment may include alcohol dehydrogenase inhibitor (fomepizole, Antizol®) and ethanol Fomepizole and ethanol both compete with methanol for binding alcohol dehydrogenase. ...
... Methanol is toxic – 10 mL can cause blindness due to its metabolite formic acid. Treatment may include alcohol dehydrogenase inhibitor (fomepizole, Antizol®) and ethanol Fomepizole and ethanol both compete with methanol for binding alcohol dehydrogenase. ...
Student 5
... animals and plants can live is about 40°C. Above 50°C the only organisms that can survive the heat are some groups of bacteria and archaea. A thermophile is an organism that thrives at relatively high temperatures, between 45 and 80 °C. Many thermophiles are archaea. It has been suggested that therm ...
... animals and plants can live is about 40°C. Above 50°C the only organisms that can survive the heat are some groups of bacteria and archaea. A thermophile is an organism that thrives at relatively high temperatures, between 45 and 80 °C. Many thermophiles are archaea. It has been suggested that therm ...
File - Manthey AP Biology
... they can happen quickly or slowly For a process to occur without energy input, it must increase the entropy of the universe ...
... they can happen quickly or slowly For a process to occur without energy input, it must increase the entropy of the universe ...
Chapter 8 Metabolism APc8metabolismme (1)
... they can happen quickly or slowly For a process to occur without energy input, it must increase the entropy of the universe ...
... they can happen quickly or slowly For a process to occur without energy input, it must increase the entropy of the universe ...
Lecture 3 (BY 14)
... • Atoms or clusters of atoms that are covalently bonded to carbon backbone • Give organic compounds their different properties ...
... • Atoms or clusters of atoms that are covalently bonded to carbon backbone • Give organic compounds their different properties ...
Enzyme

Enzymes /ˈɛnzaɪmz/ are macromolecular biological catalysts. Enzymes accelerate, or catalyze, chemical reactions. The molecules at the beginning of the process are called substrates and the enzyme converts these into different molecules, called products. Almost all metabolic processes in the cell need enzymes in order to occur at rates fast enough to sustain life. The set of enzymes made in a cell determines which metabolic pathways occur in that cell. The study of enzymes is called enzymology.Enzymes are known to catalyze more than 5,000 biochemical reaction types. Most enzymes are proteins, although a few are catalytic RNA molecules. Enzymes' specificity comes from their unique three-dimensional structures.Like all catalysts, enzymes increase the rate of a reaction by lowering its activation energy. Some enzymes can make their conversion of substrate to product occur many millions of times faster. An extreme example is orotidine 5'-phosphate decarboxylase, which allows a reaction that would otherwise take millions of years to occur in milliseconds. Chemically, enzymes are like any catalyst and are not consumed in chemical reactions, nor do they alter the equilibrium of a reaction. Enzymes differ from most other catalysts by being much more specific. Enzyme activity can be affected by other molecules: inhibitors are molecules that decrease enzyme activity, and activators are molecules that increase activity. Many drugs and poisons are enzyme inhibitors. An enzyme's activity decreases markedly outside its optimal temperature and pH.Some enzymes are used commercially, for example, in the synthesis of antibiotics. Some household products use enzymes to speed up chemical reactions: enzymes in biological washing powders break down protein, starch or fat stains on clothes, and enzymes in meat tenderizer break down proteins into smaller molecules, making the meat easier to chew.