2.3 Carbon-Based Molecules
... • Proteins are polymers of amino acid monomers. – Twenty different amino acids are used to build proteins in organisms. – Amino acids differ in side groups, or R groups. – Amino acids are linked by peptide bonds. ...
... • Proteins are polymers of amino acid monomers. – Twenty different amino acids are used to build proteins in organisms. – Amino acids differ in side groups, or R groups. – Amino acids are linked by peptide bonds. ...
Fermentation - mvhs
... Fermentation • Occurs when there is no oxygen available • allows some cells to produce ATP without the use of oxygen – ATP yield would be lower, though. Do you know why? – Only glycolysis is carried out– only 2 ATP produced. ...
... Fermentation • Occurs when there is no oxygen available • allows some cells to produce ATP without the use of oxygen – ATP yield would be lower, though. Do you know why? – Only glycolysis is carried out– only 2 ATP produced. ...
The Enolase Superfamily: A General Strategy for Enzyme
... We have discovered a superfamily of enzymes related by their ability to catalyze the abstraction of the R-proton of a carboxylic acid to form an enolic intermediate. Although each reaction catalyzed by these enzymes is initiated by this common step, their overall reactions (including racemization, β ...
... We have discovered a superfamily of enzymes related by their ability to catalyze the abstraction of the R-proton of a carboxylic acid to form an enolic intermediate. Although each reaction catalyzed by these enzymes is initiated by this common step, their overall reactions (including racemization, β ...
energy, cellular respiration
... Redox reactions are linked oxidations and reductions • Glucose gives up energy as it is oxidized oxidation = loss of H Oxygen is reduced (gains H) Loss of hydrogen atoms ...
... Redox reactions are linked oxidations and reductions • Glucose gives up energy as it is oxidized oxidation = loss of H Oxygen is reduced (gains H) Loss of hydrogen atoms ...
energy, cellular respiration
... Redox reactions are linked oxidations and reductions • Glucose gives up energy as it is oxidized oxidation = loss of H Oxygen is reduced (gains H) Loss of hydrogen atoms ...
... Redox reactions are linked oxidations and reductions • Glucose gives up energy as it is oxidized oxidation = loss of H Oxygen is reduced (gains H) Loss of hydrogen atoms ...
2.3 Carbon-Based Molecules
... 2.3 Carbon-Based Molecules • Carbohydrates can be broken down to provide energy for cells. • Some carbohydrates are part of cell structure. Polymer (starch) Starch is a polymer of glucose monomers that often has a branched structure. ...
... 2.3 Carbon-Based Molecules • Carbohydrates can be broken down to provide energy for cells. • Some carbohydrates are part of cell structure. Polymer (starch) Starch is a polymer of glucose monomers that often has a branched structure. ...
2.3 Carbon-Based Molecules KEY CONCEPT Carbon-based molecules are the foundation of life.
... 2.3 Carbon-Based Molecules • Carbohydrates can be broken down to provide energy for cells. • Some carbohydrates are part of cell structure. Polymer (starch) Starch is a polymer of glucose monomers that often has a branched structure. ...
... 2.3 Carbon-Based Molecules • Carbohydrates can be broken down to provide energy for cells. • Some carbohydrates are part of cell structure. Polymer (starch) Starch is a polymer of glucose monomers that often has a branched structure. ...
pharmaceutical biochemistry
... the aldehyde group is conserved by formation of the acid anhydride with phosphoric acid while NAD is reduced to NADH. The active site of the enzyme contains an –SH group (Cys residue) and it can be inhibited by monoiodoacetate. Arsenate toxicity is based on this reaction as well: arsenate is structu ...
... the aldehyde group is conserved by formation of the acid anhydride with phosphoric acid while NAD is reduced to NADH. The active site of the enzyme contains an –SH group (Cys residue) and it can be inhibited by monoiodoacetate. Arsenate toxicity is based on this reaction as well: arsenate is structu ...
Lehninger Principles of Biochemistry 5/e
... AMP concentration is more sensitive indicator of cell’s energetic state than is [ATP] AMP-activated protein kinase - regulated by [AMP] - A reduced nutrient supply or by increase exercise cause the rise in [AMP] - increase glucose uptake, activates glycolysis and fatty acid oxidation - suppress ener ...
... AMP concentration is more sensitive indicator of cell’s energetic state than is [ATP] AMP-activated protein kinase - regulated by [AMP] - A reduced nutrient supply or by increase exercise cause the rise in [AMP] - increase glucose uptake, activates glycolysis and fatty acid oxidation - suppress ener ...
Chajlter 31
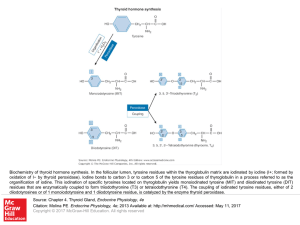
... activity, which depends on the presence of metal ions at the active site of the enzyme or in a key coenzyme. Of the latter, the best known is vitamin B12 , which contains Co. Important metalloenzymes include carboxypep tidase (Zn), alcohol dehydrogenase (Zn), superoxide dismutase (Cu, Zn), urease ( ...
... activity, which depends on the presence of metal ions at the active site of the enzyme or in a key coenzyme. Of the latter, the best known is vitamin B12 , which contains Co. Important metalloenzymes include carboxypep tidase (Zn), alcohol dehydrogenase (Zn), superoxide dismutase (Cu, Zn), urease ( ...
2.3 Carbon-Based Molecules
... 2.3 Carbon-Based Molecules • Carbohydrates can be broken down to provide energy for cells. • Some carbohydrates are part of cell structure. Polymer (starch) Starch is a polymer of glucose monomers that often has a branched structure. ...
... 2.3 Carbon-Based Molecules • Carbohydrates can be broken down to provide energy for cells. • Some carbohydrates are part of cell structure. Polymer (starch) Starch is a polymer of glucose monomers that often has a branched structure. ...
PDF
... Methodology/Principal Findings. The whole genome sequence of the diatom Phaeodactylum tricornutum has recently been completed. We identified and annotated genes for enzymes involved in carbohydrate pathways based on extensive EST support and comparison to the whole genome sequence of a second diatom ...
... Methodology/Principal Findings. The whole genome sequence of the diatom Phaeodactylum tricornutum has recently been completed. We identified and annotated genes for enzymes involved in carbohydrate pathways based on extensive EST support and comparison to the whole genome sequence of a second diatom ...
Regulation of phospholipase D activity, membrane targeting and
... vesicles, with an additional specific increase in binding affinity observed when PtdIns(4,5)P2 is included in the vesicles [16]. A similar PtdIns(4,5)P2-dependent increase in binding affinity was exhibited by an enzymatically active mutant of PLD1 lacking the N-terminus and inter catalytic domain ‘l ...
... vesicles, with an additional specific increase in binding affinity observed when PtdIns(4,5)P2 is included in the vesicles [16]. A similar PtdIns(4,5)P2-dependent increase in binding affinity was exhibited by an enzymatically active mutant of PLD1 lacking the N-terminus and inter catalytic domain ‘l ...
amino acids - CRCBiologyY11
... hydrogen, oxygen and nitrogen. They sometimes also contain sulphur and may form complexes with other molecules. Proteins are made of small units called amino acids. These link together by peptide bonds to form chains of polypeptides. Musical Proteins ...
... hydrogen, oxygen and nitrogen. They sometimes also contain sulphur and may form complexes with other molecules. Proteins are made of small units called amino acids. These link together by peptide bonds to form chains of polypeptides. Musical Proteins ...
【金屬鍵】
... In an investigation, the rate at which starch was broken down by the enzyme amylase was measured in solutions with different concentrations of starch. The experiment was carried out at 25ºC and was repeated with mercury ions added to the starch solutions. The graph shows the results. ...
... In an investigation, the rate at which starch was broken down by the enzyme amylase was measured in solutions with different concentrations of starch. The experiment was carried out at 25ºC and was repeated with mercury ions added to the starch solutions. The graph shows the results. ...
Fatty Acids
... Critical Thinking Question: During chemistry lab, Maria places sucrose (table sugar) in a glass beaker, adds water and stirs. As the table sugar disappears, she loudly proclaims that she has chemically broken down the sucrose into fructose and glucose. Is Maria’s chemical analysis ...
... Critical Thinking Question: During chemistry lab, Maria places sucrose (table sugar) in a glass beaker, adds water and stirs. As the table sugar disappears, she loudly proclaims that she has chemically broken down the sucrose into fructose and glucose. Is Maria’s chemical analysis ...
The citric acid cycle is the
... transformation of acetyl-CoA to oxaloacetate. Thus, for every succinate that enters the reversed cycle, two succinates are returned, making the cycle highly autocatalytic. • Because TCA cycle intermediates are involved in many biosynthetic pathways, a reversed TCA cycle would be a bountifuland broad ...
... transformation of acetyl-CoA to oxaloacetate. Thus, for every succinate that enters the reversed cycle, two succinates are returned, making the cycle highly autocatalytic. • Because TCA cycle intermediates are involved in many biosynthetic pathways, a reversed TCA cycle would be a bountifuland broad ...
Krebs cycle - biology.org.uk
... joins with oxaloacetate, a four-carbon compound, to form citrate, a six-carbon compound 2 Citrate is decarboxylated (one molecule of CO 2 removed) and dehydrogenated (two hydrogen atoms removed) to form a five-carbon compound; and the hydrogen atoms are accepted by an NAD molecule, which gets reduce ...
... joins with oxaloacetate, a four-carbon compound, to form citrate, a six-carbon compound 2 Citrate is decarboxylated (one molecule of CO 2 removed) and dehydrogenated (two hydrogen atoms removed) to form a five-carbon compound; and the hydrogen atoms are accepted by an NAD molecule, which gets reduce ...
Partial purification of fatty acid synthetase from Streptomyces
... Thus the synthetase migrated as a single species during these Filamentous bacteria of the genus Streptomyces are extremely procedures (with a consistent recovery of over 80%), and its versatile in making antibiotics, many of which are phenolic activity presumably resides in multifunctional polypepti ...
... Thus the synthetase migrated as a single species during these Filamentous bacteria of the genus Streptomyces are extremely procedures (with a consistent recovery of over 80%), and its versatile in making antibiotics, many of which are phenolic activity presumably resides in multifunctional polypepti ...
Enzyme

Enzymes /ˈɛnzaɪmz/ are macromolecular biological catalysts. Enzymes accelerate, or catalyze, chemical reactions. The molecules at the beginning of the process are called substrates and the enzyme converts these into different molecules, called products. Almost all metabolic processes in the cell need enzymes in order to occur at rates fast enough to sustain life. The set of enzymes made in a cell determines which metabolic pathways occur in that cell. The study of enzymes is called enzymology.Enzymes are known to catalyze more than 5,000 biochemical reaction types. Most enzymes are proteins, although a few are catalytic RNA molecules. Enzymes' specificity comes from their unique three-dimensional structures.Like all catalysts, enzymes increase the rate of a reaction by lowering its activation energy. Some enzymes can make their conversion of substrate to product occur many millions of times faster. An extreme example is orotidine 5'-phosphate decarboxylase, which allows a reaction that would otherwise take millions of years to occur in milliseconds. Chemically, enzymes are like any catalyst and are not consumed in chemical reactions, nor do they alter the equilibrium of a reaction. Enzymes differ from most other catalysts by being much more specific. Enzyme activity can be affected by other molecules: inhibitors are molecules that decrease enzyme activity, and activators are molecules that increase activity. Many drugs and poisons are enzyme inhibitors. An enzyme's activity decreases markedly outside its optimal temperature and pH.Some enzymes are used commercially, for example, in the synthesis of antibiotics. Some household products use enzymes to speed up chemical reactions: enzymes in biological washing powders break down protein, starch or fat stains on clothes, and enzymes in meat tenderizer break down proteins into smaller molecules, making the meat easier to chew.