Interpretive Guide for Amino Acids
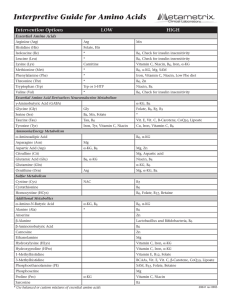
... α-Amino-N-butyric acid Low - possible increased need for the nutrients which aid in threonine metabolism from which this AA is derived. These include α-KG and B6. High - inadequate utilization of this AA for cellular energy generation. Alpha-ABA is converted to succinyl Co-A for use in the citric ac ...
... α-Amino-N-butyric acid Low - possible increased need for the nutrients which aid in threonine metabolism from which this AA is derived. These include α-KG and B6. High - inadequate utilization of this AA for cellular energy generation. Alpha-ABA is converted to succinyl Co-A for use in the citric ac ...
Phenylpropanoids
... The term, proanthocyanidins, is derived from the acid catalyzed oxidation reaction that produces red anthocyanidins upon heating PAs in acidic alcohol ...
... The term, proanthocyanidins, is derived from the acid catalyzed oxidation reaction that produces red anthocyanidins upon heating PAs in acidic alcohol ...
are PROTEINS!!!!!!
... • The two types of nucleic acids, deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), are composed of chains of nucleotides. • Nucleotides consist of a sugar, a phosphate group, and a nitrogen-containing base. ...
... • The two types of nucleic acids, deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), are composed of chains of nucleotides. • Nucleotides consist of a sugar, a phosphate group, and a nitrogen-containing base. ...
DNA and Proteins
... place at the ribosomes. • The process of converting the information in mRNA into a sequence of amino acids in a protein. ...
... place at the ribosomes. • The process of converting the information in mRNA into a sequence of amino acids in a protein. ...
HERE
... o α-Amino groups of most amino acids are ultimately transferred to αOxoglutarate by Transamination, forming Glutamate, the Amino group is then removed as Ammonia by Oxidation ...
... o α-Amino groups of most amino acids are ultimately transferred to αOxoglutarate by Transamination, forming Glutamate, the Amino group is then removed as Ammonia by Oxidation ...
Photosynthesis Modeling Activity
... cellulose, which are polymers of glucose. Other glucose molecules go on to cellular respiration which creates useable energy for the cells (ATP) from glucose. The sugars produced by photosynthesis are also used to make other plant molecules such as the amino acids which are the building blocks for p ...
... cellulose, which are polymers of glucose. Other glucose molecules go on to cellular respiration which creates useable energy for the cells (ATP) from glucose. The sugars produced by photosynthesis are also used to make other plant molecules such as the amino acids which are the building blocks for p ...
Carbohydrate Metabolism of Staphylococcus aureus
... After adding non-radioactive glucose, filtrate and wash-waters were oxidized with K,S,O, (Aronoff, 1960) and the CO, was collected as BaCO,. The BaCO, was handled as described by Bosch (1955) and the radioactivity was determined on a layer of infinite thickness, using an end-window GM tube. The cocc ...
... After adding non-radioactive glucose, filtrate and wash-waters were oxidized with K,S,O, (Aronoff, 1960) and the CO, was collected as BaCO,. The BaCO, was handled as described by Bosch (1955) and the radioactivity was determined on a layer of infinite thickness, using an end-window GM tube. The cocc ...
Master Beekeeper Certification Course: Category #7
... the stored nectar. This raw honey is composed of a variety of sugars; mainly glucose (dextrose), fructose (levulose), sucrose and maltose, however there are many other chemicals and substances in honey which contribute to its taste, color, pH, viscosity and aroma. Some of these chemicals aid in the ...
... the stored nectar. This raw honey is composed of a variety of sugars; mainly glucose (dextrose), fructose (levulose), sucrose and maltose, however there are many other chemicals and substances in honey which contribute to its taste, color, pH, viscosity and aroma. Some of these chemicals aid in the ...
Choose the response which best completes each of the following
... the(1.) Golgi apparatus (2.) centriole (3.) nucleus (4.) endoplasmic reticulum (5.) mitochondrion 11. A student examining a cell under the microscope noticed the formation of a cell plate in the midline of the cell and the formation of nuclei at the poles of the cell. The cell under examination was ...
... the(1.) Golgi apparatus (2.) centriole (3.) nucleus (4.) endoplasmic reticulum (5.) mitochondrion 11. A student examining a cell under the microscope noticed the formation of a cell plate in the midline of the cell and the formation of nuclei at the poles of the cell. The cell under examination was ...
Cancer_JC_presentation_2009
... constitutive signaling through EGFR and PI3K, and it depends on flux through the PPP • Antioxidants can reverse the metabolic defect, independent of glucose uptake, by increasing flux through the PPP • Antioxidants can enhance the transforming activity of oncogenic cells. ...
... constitutive signaling through EGFR and PI3K, and it depends on flux through the PPP • Antioxidants can reverse the metabolic defect, independent of glucose uptake, by increasing flux through the PPP • Antioxidants can enhance the transforming activity of oncogenic cells. ...
A INSTRUCTIONS
... Massive doses of methylene blue are sometimes given for cyanide poisoning. Which one of the following statements is INCORRECT? (A) Reduction potential of methylene blue is similar to oxygen (B) Cyanide blocks transfer of electrons from cytochrome oxidase to oxygen (C) In cyanide poisoning, all the r ...
... Massive doses of methylene blue are sometimes given for cyanide poisoning. Which one of the following statements is INCORRECT? (A) Reduction potential of methylene blue is similar to oxygen (B) Cyanide blocks transfer of electrons from cytochrome oxidase to oxygen (C) In cyanide poisoning, all the r ...
Nucleotides: Synthesis and Degredation
... Step 1: synthesis of carbamoyl phosphate Condensation of glutamine, bicarbonate in the presence of ATP Carbamoyl phosphate synthetase exists in 2 types: CPS-I which is a mitochondrial enzyme and is dedicated to the urea cycle and arginine biosynthesis) and CPS-II, a cytosolic enzyme ...
... Step 1: synthesis of carbamoyl phosphate Condensation of glutamine, bicarbonate in the presence of ATP Carbamoyl phosphate synthetase exists in 2 types: CPS-I which is a mitochondrial enzyme and is dedicated to the urea cycle and arginine biosynthesis) and CPS-II, a cytosolic enzyme ...
Organic Compounds
... atoms to Oxygen much higher than in carbohydrates, (this is why lipids contains more energy than carbohydrates). The most important molecules of lipids are the fats. Fats are composed of a glycerol molecule (that contains hydroxyl group -OH), and one, two or three units of fatty acid tales (a long c ...
... atoms to Oxygen much higher than in carbohydrates, (this is why lipids contains more energy than carbohydrates). The most important molecules of lipids are the fats. Fats are composed of a glycerol molecule (that contains hydroxyl group -OH), and one, two or three units of fatty acid tales (a long c ...
PPT
... • Cytochrome oxidase catalyzes the reduction of a final electron acceptor, oxygen • An artifcial e- donor, phenylenediamine, is used to reduce the cytochrome oxidase • If the enzyme is present, the colorless reagent (reduced state) will turn blue (oxidized state) ...
... • Cytochrome oxidase catalyzes the reduction of a final electron acceptor, oxygen • An artifcial e- donor, phenylenediamine, is used to reduce the cytochrome oxidase • If the enzyme is present, the colorless reagent (reduced state) will turn blue (oxidized state) ...
k28 The hydrogen hypothesis for the first eukaryote - e
... came along,” writes Thomas B. L. Kirkwood.7 The planet’s early dominant life form, namely photosynthetic bacteria, is the usual villain cited for excreting the “toxic” oxygen. But before this, defenses would long have been, suggests Nick Lane in Oxygen: The Molecule that Made the World, 2003, becaus ...
... came along,” writes Thomas B. L. Kirkwood.7 The planet’s early dominant life form, namely photosynthetic bacteria, is the usual villain cited for excreting the “toxic” oxygen. But before this, defenses would long have been, suggests Nick Lane in Oxygen: The Molecule that Made the World, 2003, becaus ...
BIOCHEMISTRY NOTES
... inactivated 2. These interactions occur among enzymes that have at least 2 binding sites, one an the active site and another, into which a second molecule (called the allosteric effector) fits. 3. The binding of the allosteric effector changes the shape of the enzyme molecule and either temporarily ...
... inactivated 2. These interactions occur among enzymes that have at least 2 binding sites, one an the active site and another, into which a second molecule (called the allosteric effector) fits. 3. The binding of the allosteric effector changes the shape of the enzyme molecule and either temporarily ...
Organic Chemistry – Review #2 Vocabulary Adhesion Cohesion
... A. large amount of stored information B. ability to catalyze biochemical reactions C. efficient storage of usable chemical energy D. tendency to make cell membranes hydrophobic 4. Substance A is converted to substance B in a metabolic reaction. Which statement best describes the role of an enzyme du ...
... A. large amount of stored information B. ability to catalyze biochemical reactions C. efficient storage of usable chemical energy D. tendency to make cell membranes hydrophobic 4. Substance A is converted to substance B in a metabolic reaction. Which statement best describes the role of an enzyme du ...
Curriculum for Excellence Higher Chemistry Unit 2 Nature`s Che
... The hydrocarbon tail is hydrophobic (water-hating) and dissolves well in grease and oil. How to explain the cleaning action of soap. Hard water contains calcium and magnesium ions which form a scum with soap. Detergents were developed to combat hard-water conditions. Detergents clean in th ...
... The hydrocarbon tail is hydrophobic (water-hating) and dissolves well in grease and oil. How to explain the cleaning action of soap. Hard water contains calcium and magnesium ions which form a scum with soap. Detergents were developed to combat hard-water conditions. Detergents clean in th ...
Biology_Chapter 8_Cellular_Respiration
... oxygen also releases energy in the form of heat and light (flames) Living organisms get their energy from reactions like this (but not reactions which are violent enough to produce ...
... oxygen also releases energy in the form of heat and light (flames) Living organisms get their energy from reactions like this (but not reactions which are violent enough to produce ...
Glucose
... III. ln the stomach: carbohydrate digestion stops temporarily due to the high acidity which inactivates the salivary - amylase. IV. Digestion of carbohydrate by the pancreatic - amylase small intestine in the small intestine. A. α-amylase enzyme is produced by pancreas and acts in small intestine. ...
... III. ln the stomach: carbohydrate digestion stops temporarily due to the high acidity which inactivates the salivary - amylase. IV. Digestion of carbohydrate by the pancreatic - amylase small intestine in the small intestine. A. α-amylase enzyme is produced by pancreas and acts in small intestine. ...
Monosaccharides
... having more chiral atoms. For example, aldohexoses (С6Н12О6), that contain 4 chiral atoms, have 16 stereoisimers (N=2n) and 8 pair of enantiomers To determine the belonging of monosaccharide to L- or D-series we use the configuration at the farthest carbon atom from the ...
... having more chiral atoms. For example, aldohexoses (С6Н12О6), that contain 4 chiral atoms, have 16 stereoisimers (N=2n) and 8 pair of enantiomers To determine the belonging of monosaccharide to L- or D-series we use the configuration at the farthest carbon atom from the ...
2004 Lec 42-43: Nucleotide Metabolism
... allopurinol treatment of gout and LeschNyhan syndromes. ©Copyright 1999-2004 by Gene C. Lavers, Ph.D. ...
... allopurinol treatment of gout and LeschNyhan syndromes. ©Copyright 1999-2004 by Gene C. Lavers, Ph.D. ...
UNIT 5 I. Energy and the Cell Module 5.1 Energy is the capacity to
... toxic than alcohol and can be removed from the affected cells and detoxified by the liver with the Cori cycle (Figure 6.13A). C. Alcoholic fermentation, characteristic of some yeasts and bacteria, results in one two-carbon ethanol. This product is toxic, and high concentrations will ultimately kill ...
... toxic than alcohol and can be removed from the affected cells and detoxified by the liver with the Cori cycle (Figure 6.13A). C. Alcoholic fermentation, characteristic of some yeasts and bacteria, results in one two-carbon ethanol. This product is toxic, and high concentrations will ultimately kill ...
Citric acid cycle
The citric acid cycle – also known as the tricarboxylic acid (TCA) cycle or the Krebs cycle – is a series of chemical reactions used by all aerobic organisms to generate energy through the oxidation of acetate derived from carbohydrates, fats and proteins into carbon dioxide and chemical energy in the form of adenosine triphosphate (ATP). In addition, the cycle provides precursors of certain amino acids as well as the reducing agent NADH that is used in numerous other biochemical reactions. Its central importance to many biochemical pathways suggests that it was one of the earliest established components of cellular metabolism and may have originated abiogenically.The name of this metabolic pathway is derived from citric acid (a type of tricarboxylic acid) that is consumed and then regenerated by this sequence of reactions to complete the cycle. In addition, the cycle consumes acetate (in the form of acetyl-CoA) and water, reduces NAD+ to NADH, and produces carbon dioxide as a waste byproduct. The NADH generated by the TCA cycle is fed into the oxidative phosphorylation (electron transport) pathway. The net result of these two closely linked pathways is the oxidation of nutrients to produce usable chemical energy in the form of ATP.In eukaryotic cells, the citric acid cycle occurs in the matrix of the mitochondrion. In prokaryotic cells, such as bacteria which lack mitochondria, the TCA reaction sequence is performed in the cytosol with the proton gradient for ATP production being across the cell's surface (plasma membrane) rather than the inner membrane of the mitochondrion.























