Enzymes
... • [S] generally < than its Km – Only uses fraction of enzyme catalytic ability – Enzyme is able to respond to changes in [S] ...
... • [S] generally < than its Km – Only uses fraction of enzyme catalytic ability – Enzyme is able to respond to changes in [S] ...
The Structure and Function of Macromolecules
... • Use the triangle water to point to the bond site. Draw an arrow to show if water is being added or released during this reaction. Label as a 4-monomer polypeptide ...
... • Use the triangle water to point to the bond site. Draw an arrow to show if water is being added or released during this reaction. Label as a 4-monomer polypeptide ...
Energy Metabolism - Rajarata University of Sri Lanka
... are obtained by breaking down organic substrates obtained from the environment, through catabolic pathways, so releasing chemically available energy (i.e. ATP) and/or generating metabolic intermediates used in anabolic pathways18. Although there are at least 30 different amino acids described in nat ...
... are obtained by breaking down organic substrates obtained from the environment, through catabolic pathways, so releasing chemically available energy (i.e. ATP) and/or generating metabolic intermediates used in anabolic pathways18. Although there are at least 30 different amino acids described in nat ...
Amino Acids and Simple Proteins
... Hydrolyzable lipids. Non-hydrolyzable lipids. Biological roles. Fatty acids and fats. Structure of phospholipids and glycolipids. Isoprenoids. Sterols. Steroid hormones. Bile acids. 5. Energy metabolism Catabolism and anabolism. Methods of metabolism investigations. Types of metabolism and its regul ...
... Hydrolyzable lipids. Non-hydrolyzable lipids. Biological roles. Fatty acids and fats. Structure of phospholipids and glycolipids. Isoprenoids. Sterols. Steroid hormones. Bile acids. 5. Energy metabolism Catabolism and anabolism. Methods of metabolism investigations. Types of metabolism and its regul ...
Towards biome-specific analysis of meta-omics data
... current studies usually rely on broad metabolic databases (for example, KEGG (Kanehisa et al., 2014)). Despite their unquestionable merit, such resources unfortunately tend to be biased, for historical reasons, toward Eukaryotes and model organisms’ metabolism. These databases thus often include pat ...
... current studies usually rely on broad metabolic databases (for example, KEGG (Kanehisa et al., 2014)). Despite their unquestionable merit, such resources unfortunately tend to be biased, for historical reasons, toward Eukaryotes and model organisms’ metabolism. These databases thus often include pat ...
Biochem09 - Amit Kessel Ph.D
... D. Regulation of the catalytic activities of enzymes. E. Alteration of the amounts of allosteric modifiers (regulator molecules). ...
... D. Regulation of the catalytic activities of enzymes. E. Alteration of the amounts of allosteric modifiers (regulator molecules). ...
Bio 2 – Vocabulary--Biological Molecules
... Understand the difference between fats and oils Know the structure and purpose of phospholipids Know the basic structure of a steroid Proteins Know and be able to draw the formation of a peptide bond Understand what denaturing is and how it happens Realize what makes all amino acids the ...
... Understand the difference between fats and oils Know the structure and purpose of phospholipids Know the basic structure of a steroid Proteins Know and be able to draw the formation of a peptide bond Understand what denaturing is and how it happens Realize what makes all amino acids the ...
ACID - SchoolNotes
... • In 1923, proposed an even broader definition of acids and bases. • Lewis focused on electron transfer instead of proton transfer. • He defined and acid as an electron-pair acceptor, and a base as an electron-pair donor. • This definition applies to solutions and reactions which do not even involve ...
... • In 1923, proposed an even broader definition of acids and bases. • Lewis focused on electron transfer instead of proton transfer. • He defined and acid as an electron-pair acceptor, and a base as an electron-pair donor. • This definition applies to solutions and reactions which do not even involve ...
Triacylglycerol Metabolism Gone Bad: A major cause of disease
... and chylomicrons by the action of Lipoprotein lipase. – L.P. lipase is an extracellular enzyme, located on the endothelial wall. It is teathered to heparin. – L.P. Lipase is made in adipocytes (as well as other cells). Insulin stimulates the secretion of L.P. Lipase. ...
... and chylomicrons by the action of Lipoprotein lipase. – L.P. lipase is an extracellular enzyme, located on the endothelial wall. It is teathered to heparin. – L.P. Lipase is made in adipocytes (as well as other cells). Insulin stimulates the secretion of L.P. Lipase. ...
Chapter 13 (part 1) - University of Nevada, Reno
... – Reciprocal regulation must turn one on and the other off - this requires something new! ...
... – Reciprocal regulation must turn one on and the other off - this requires something new! ...
Chapter Fourteen: Metabolism: Basic Concepts and
... 38. How much ATP is used daily by a typical human? How is it regenerated? Answer: A human uses 40 kg of ATP per day. There is only about 100 g ATP available, thus the ATP is used and regenerated rapidly. ATP is regenerated from ADP and Pi, using the energy from catabolic processes. 39. What is an io ...
... 38. How much ATP is used daily by a typical human? How is it regenerated? Answer: A human uses 40 kg of ATP per day. There is only about 100 g ATP available, thus the ATP is used and regenerated rapidly. ATP is regenerated from ADP and Pi, using the energy from catabolic processes. 39. What is an io ...
Slide 12
... shape >> cyclic ring + extra 3 acids ) . -The ring formed because number 1 amino acid and number 6 amino acid are cysteine and they can form disulfide bond with each other . *Oxytocin : -Secreted from posterior pituitary gland , it's receptors found in the uterine wall and in breasts -receports incr ...
... shape >> cyclic ring + extra 3 acids ) . -The ring formed because number 1 amino acid and number 6 amino acid are cysteine and they can form disulfide bond with each other . *Oxytocin : -Secreted from posterior pituitary gland , it's receptors found in the uterine wall and in breasts -receports incr ...
Chapter 3 Lecture notes
... obtain glucose. NOTE: The unbranched form of starch is called amylose. The branched form is called amylopectin. Starches rich in amylopectin retain water and are often used in frozen foods. This can also be used to illustrate the convention used for naming enzymes— the starch amylose is broken down ...
... obtain glucose. NOTE: The unbranched form of starch is called amylose. The branched form is called amylopectin. Starches rich in amylopectin retain water and are often used in frozen foods. This can also be used to illustrate the convention used for naming enzymes— the starch amylose is broken down ...
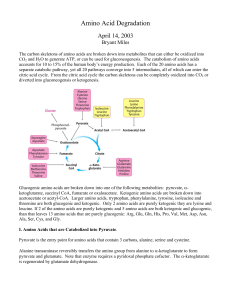
Amino Acid Degradation
... CO2 and H2O to generate ATP, or can be used for gluconeogenesis. The catabolism of amino acids accounts for 10 to 15% of the human body’s energy production. Each of the 20 amino acids has a separate catabolic pathway, yet all 20 pathways converge into 5 intermediates, all of which can enter the citr ...
... CO2 and H2O to generate ATP, or can be used for gluconeogenesis. The catabolism of amino acids accounts for 10 to 15% of the human body’s energy production. Each of the 20 amino acids has a separate catabolic pathway, yet all 20 pathways converge into 5 intermediates, all of which can enter the citr ...
lecture ppt
... Termites have bacteria that can break down cellulose Cellulose β-glucose, with the -OH pointed out Starch α-glucose, with the -OH pointed down ...
... Termites have bacteria that can break down cellulose Cellulose β-glucose, with the -OH pointed out Starch α-glucose, with the -OH pointed down ...
Model Description Sheet
... Antibiotic-resistant bacteria are common and hard to treat. There is potential to create synthetic antibiotics based on natural products like enduracidin and mannopeptimycin to fight drug resistant bacteria like MRSA. MppP, an enzyme from Streptomyces wadayamensis, is required for the biosynthesis o ...
... Antibiotic-resistant bacteria are common and hard to treat. There is potential to create synthetic antibiotics based on natural products like enduracidin and mannopeptimycin to fight drug resistant bacteria like MRSA. MppP, an enzyme from Streptomyces wadayamensis, is required for the biosynthesis o ...
Mechanisms of Enzymes
... state free energy. - Result of Acid/Base catalysis is making a reactive group more reactive by increasing its intrinsic electrophilic or nucleophilic character - This can increase the rate 10-100 fold - Microenvironment shifts in pKa allow for several amino acids to be involved: Asp, Glu, His, Cys, ...
... state free energy. - Result of Acid/Base catalysis is making a reactive group more reactive by increasing its intrinsic electrophilic or nucleophilic character - This can increase the rate 10-100 fold - Microenvironment shifts in pKa allow for several amino acids to be involved: Asp, Glu, His, Cys, ...
Enzymes, ATP and Bioenergetics
... Note – Coenzymes in their reduced form (NADH + H+ and FADH2) have a higher energy potential than they do when in their oxidized form (NAD and FAD). 3. Constitutive Vs repressible Vs inducible enzymes - Enzymes can be categorized as constitutive, repressible or inducible on the basis of regulatory m ...
... Note – Coenzymes in their reduced form (NADH + H+ and FADH2) have a higher energy potential than they do when in their oxidized form (NAD and FAD). 3. Constitutive Vs repressible Vs inducible enzymes - Enzymes can be categorized as constitutive, repressible or inducible on the basis of regulatory m ...
Transcription, Translation, and Protein Study Guide What is the
... 6th position of the beta subunit of the hemoglobin molecule can alters the shape of hemoglobin when in a low oxygen or stress environment. Hemoglobin is a quaternary protein made of 4 tertiary subunits, 2 alpha and 2 beta, held together by a central molecule of iron. Identify an amino acid structure ...
... 6th position of the beta subunit of the hemoglobin molecule can alters the shape of hemoglobin when in a low oxygen or stress environment. Hemoglobin is a quaternary protein made of 4 tertiary subunits, 2 alpha and 2 beta, held together by a central molecule of iron. Identify an amino acid structure ...
Dionex AminoPac Columns for the Analysis of Amino Acids
... protein of interest, and the choice of hydrolysis procedures is key to accurate analysis as some sensitive amino acids may be destroyed during the hydrolysis. • After hydrolysis, the hydrolyzing reagents are removed (typically by evaporation) and the hydrosylate is reconstituted in water or oth ...
... protein of interest, and the choice of hydrolysis procedures is key to accurate analysis as some sensitive amino acids may be destroyed during the hydrolysis. • After hydrolysis, the hydrolyzing reagents are removed (typically by evaporation) and the hydrosylate is reconstituted in water or oth ...
Cellular Respiration
... a. What provided the spark to start the candle burning? b. What provides the fuel for the burning candle? 2. Is the burning candle giving off any type of energy? If so, what kind(s) of energy are being released? 3. Place the beaker or flask over the candle. What happens? 4. What caused the candle to ...
... a. What provided the spark to start the candle burning? b. What provides the fuel for the burning candle? 2. Is the burning candle giving off any type of energy? If so, what kind(s) of energy are being released? 3. Place the beaker or flask over the candle. What happens? 4. What caused the candle to ...
Citric acid cycle
The citric acid cycle – also known as the tricarboxylic acid (TCA) cycle or the Krebs cycle – is a series of chemical reactions used by all aerobic organisms to generate energy through the oxidation of acetate derived from carbohydrates, fats and proteins into carbon dioxide and chemical energy in the form of adenosine triphosphate (ATP). In addition, the cycle provides precursors of certain amino acids as well as the reducing agent NADH that is used in numerous other biochemical reactions. Its central importance to many biochemical pathways suggests that it was one of the earliest established components of cellular metabolism and may have originated abiogenically.The name of this metabolic pathway is derived from citric acid (a type of tricarboxylic acid) that is consumed and then regenerated by this sequence of reactions to complete the cycle. In addition, the cycle consumes acetate (in the form of acetyl-CoA) and water, reduces NAD+ to NADH, and produces carbon dioxide as a waste byproduct. The NADH generated by the TCA cycle is fed into the oxidative phosphorylation (electron transport) pathway. The net result of these two closely linked pathways is the oxidation of nutrients to produce usable chemical energy in the form of ATP.In eukaryotic cells, the citric acid cycle occurs in the matrix of the mitochondrion. In prokaryotic cells, such as bacteria which lack mitochondria, the TCA reaction sequence is performed in the cytosol with the proton gradient for ATP production being across the cell's surface (plasma membrane) rather than the inner membrane of the mitochondrion.