Specific Heat Capacity of an Unknown Metal
... Chemists identify substances on the basis of their chemical and physical properties. One physical property of a substance is the amount of energy it will absorb per unit of mass. This property can be measured quite accurately and is called specific heat (cp)Specific heat is the amount of energy meas ...
... Chemists identify substances on the basis of their chemical and physical properties. One physical property of a substance is the amount of energy it will absorb per unit of mass. This property can be measured quite accurately and is called specific heat (cp)Specific heat is the amount of energy meas ...
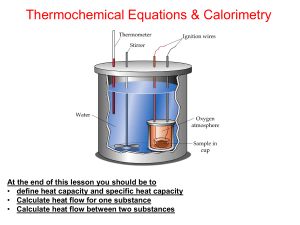
Thermochemistry Lesson 2
... At the end of this lesson you should be to • define calorimeter and calorimetry • Know five (5) ways to determine enthalpy change for a reaction • Do calculations for enthaphy of reaction using calorimetry ...
... At the end of this lesson you should be to • define calorimeter and calorimetry • Know five (5) ways to determine enthalpy change for a reaction • Do calculations for enthaphy of reaction using calorimetry ...
AP#Chemistry#Lab:#Determination#of#the#Molar#Heat#of#Fusion
... evolved!by!physical!and!chemical!processes.!Heat!(q)!can!be!defined!as!the!flow!of!energy!into!and!out!of!a! system!due!to!a!temperature!difference!between!the!thermodynamic!system!and!its!surroundings.! Heat! energy! will! flow! from! the! region! of! higher! temperature! to! the! region! of! lower ...
... evolved!by!physical!and!chemical!processes.!Heat!(q)!can!be!defined!as!the!flow!of!energy!into!and!out!of!a! system!due!to!a!temperature!difference!between!the!thermodynamic!system!and!its!surroundings.! Heat! energy! will! flow! from! the! region! of! higher! temperature! to! the! region! of! lower ...
Experiment 6 ~ Joule Heating of a Resistor
... When a resistor absorbs electrical energy, it dissipates this energy in the form of heat Q. If the resistor is placed in the calorimeter, the amount of heat produced can be measured when it is absorbed in the calorimeter. Consider the experimental arrangement shown in Figure 5.1, which a resistor co ...
... When a resistor absorbs electrical energy, it dissipates this energy in the form of heat Q. If the resistor is placed in the calorimeter, the amount of heat produced can be measured when it is absorbed in the calorimeter. Consider the experimental arrangement shown in Figure 5.1, which a resistor co ...
Adiabatic process
... Q: Energy transferred by heat to the system W: Work done on the system The first law of thermodynamics is a special case of the law of conservation of energy that relates the change in internal energy of a system to the net transfer of energy by heat and work. Internal energy is a state variable (li ...
... Q: Energy transferred by heat to the system W: Work done on the system The first law of thermodynamics is a special case of the law of conservation of energy that relates the change in internal energy of a system to the net transfer of energy by heat and work. Internal energy is a state variable (li ...
heated bar method
... The heated bar method is based on the theory of extended surfaces (fins). This apparatus can be used to calculate either the thermal conductivity of a material given the convection coefficient, h; or, h can be calculated given the thermal conductivity, k. The apparatus consists of a sample with rect ...
... The heated bar method is based on the theory of extended surfaces (fins). This apparatus can be used to calculate either the thermal conductivity of a material given the convection coefficient, h; or, h can be calculated given the thermal conductivity, k. The apparatus consists of a sample with rect ...
Heat demand for a building
... The heat demand of the air handling unit (AHU) (Formula 2.23) without heat recovery The heat demand in an AHU with the heat recovery Efficiency for heat recovery either for exhaust air or for supply air The efficiency for heat recovery for supply air (Formula 2.30) Values for temp. ratio (efficiency ...
... The heat demand of the air handling unit (AHU) (Formula 2.23) without heat recovery The heat demand in an AHU with the heat recovery Efficiency for heat recovery either for exhaust air or for supply air The efficiency for heat recovery for supply air (Formula 2.30) Values for temp. ratio (efficiency ...
Solutions - University of Illinois at Chicago
... For parts (c)-(e), replace the reservoirs by two identical but finite bodies, each characterized by a heat capacity at constant pressure C, which is independent of temperature. The bodies remain at constant pressure and undergo no change of phase. Initially, their temperatures are T1 and T2, respect ...
... For parts (c)-(e), replace the reservoirs by two identical but finite bodies, each characterized by a heat capacity at constant pressure C, which is independent of temperature. The bodies remain at constant pressure and undergo no change of phase. Initially, their temperatures are T1 and T2, respect ...
Air Temperature
... It is similar to other liquid-in-glass thermometers except that it contains a small barbell-shaped index marker in the bore. As the air temperature drops, the contracting liquid moves back into the bulb and brings the index marker down the bore with it. When the temperature stops decreasing, t ...
... It is similar to other liquid-in-glass thermometers except that it contains a small barbell-shaped index marker in the bore. As the air temperature drops, the contracting liquid moves back into the bulb and brings the index marker down the bore with it. When the temperature stops decreasing, t ...
4-Chapter-LIQUIDS-AND-SOLIDS-MCQs
... Long chain of amino acids are coiled about one another into spiral by. (a) covalent bond (b) ionic bond (c) hydrogen bond (d) Vander Waal’s forces Q.16 Evaporation of water is possible at (a) 100oC (b) 0oC (c) at all temperatures (d) above 100oC Q.17 Boiling point is low for liquid with (a) high vap ...
... Long chain of amino acids are coiled about one another into spiral by. (a) covalent bond (b) ionic bond (c) hydrogen bond (d) Vander Waal’s forces Q.16 Evaporation of water is possible at (a) 100oC (b) 0oC (c) at all temperatures (d) above 100oC Q.17 Boiling point is low for liquid with (a) high vap ...
Ch 16 Thermal Energy and Heat
... o Thermal insulator is a material that conducts thermal energy poorly o Air is a very good insulator o Wool garments and plastic foam cups are two more examples of insulators B. Convection Convection is the transfer of thermal energy when particle of a fluid move from one place to another Air ci ...
... o Thermal insulator is a material that conducts thermal energy poorly o Air is a very good insulator o Wool garments and plastic foam cups are two more examples of insulators B. Convection Convection is the transfer of thermal energy when particle of a fluid move from one place to another Air ci ...
Thermal Energy Thermal Energy Chemical Bonds Chemical Bonds
... Separates air used to burn from air in room Transfers heat efficiently without transferring particles ...
... Separates air used to burn from air in room Transfers heat efficiently without transferring particles ...
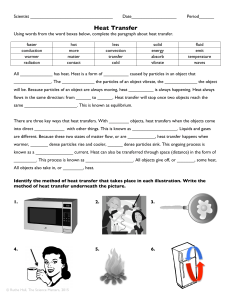
Heat Transfer - Granville County Public Schools
... All _____________ has heat. Heat is a form of __________ caused by particles in an object that _______________. The _____________ the particles of an object vibrate, the _____________ the object will be. Because particles of an object are always moving, heat __________ is always happening. Heat alwa ...
... All _____________ has heat. Heat is a form of __________ caused by particles in an object that _______________. The _____________ the particles of an object vibrate, the _____________ the object will be. Because particles of an object are always moving, heat __________ is always happening. Heat alwa ...
Ch 10 equations - mvhs
... at S.T.P.= 22.4 L Combined gas law: the pressure (P) of a fixed quantity (n) of a gas is directly proportional to the temperature (T) and inversely proportional to the volume (V). Ideal gas law: the product of pressure (P) and volume (V) is directly proportional to the product of temperature (K) and ...
... at S.T.P.= 22.4 L Combined gas law: the pressure (P) of a fixed quantity (n) of a gas is directly proportional to the temperature (T) and inversely proportional to the volume (V). Ideal gas law: the product of pressure (P) and volume (V) is directly proportional to the product of temperature (K) and ...
Document
... U (or any state function) can be expressed as an infinitesimal quantity, dU, that when integrated, depends only on the initial and final states. dU is called an exact differential. ...
... U (or any state function) can be expressed as an infinitesimal quantity, dU, that when integrated, depends only on the initial and final states. dU is called an exact differential. ...
Chapter 1.The Properties of Gases
... that permits the passage of energy as heat. An adiabatic boundary is a boundary that prevents the passage of energy as heat. • 7. Thermal equilibrium is a condition in which no change of state occurs when two objects A and B are in contact through a diathermic boundary. ...
... that permits the passage of energy as heat. An adiabatic boundary is a boundary that prevents the passage of energy as heat. • 7. Thermal equilibrium is a condition in which no change of state occurs when two objects A and B are in contact through a diathermic boundary. ...
Brewing Week 4
... stainless steel cooling coil with a 10 mm o.d., 9 mm i.d., and thermal conductivity of 100 W/m.K. The specific heat of the beer is 4.2 kJ/kg.K and the film heat transfer coefficients on the product and coolant sides are 5000 W/m2.K and 800 W/m2.K, respectively. The fouling factors on the product and ...
... stainless steel cooling coil with a 10 mm o.d., 9 mm i.d., and thermal conductivity of 100 W/m.K. The specific heat of the beer is 4.2 kJ/kg.K and the film heat transfer coefficients on the product and coolant sides are 5000 W/m2.K and 800 W/m2.K, respectively. The fouling factors on the product and ...
Chemistry Name: Random Problems Date: Hess`s Law 1. Calculate
... 3. How much heat is necessary to raise the temperature of 25.00 g of water from 10.oC to 60.oC. ...
... 3. How much heat is necessary to raise the temperature of 25.00 g of water from 10.oC to 60.oC. ...
EETopic Coversheet Word document
... Recall that nitrogen from air, and hydrogen from natural gas or the cracking of hydrocarbons, are used in the manufacture of ammonia Know that for the manufacture of ammonia by the Haber process, the essential conditions are: i) a temperature of about 450°C ii) a pressure of about 200 atmospheres ii ...
... Recall that nitrogen from air, and hydrogen from natural gas or the cracking of hydrocarbons, are used in the manufacture of ammonia Know that for the manufacture of ammonia by the Haber process, the essential conditions are: i) a temperature of about 450°C ii) a pressure of about 200 atmospheres ii ...
JIF 314 Thermodynamics
... by the amount |QH| from HTR, turning part of this heat into work |W|, and the balance of heat, |QL| =|QH| -|W|, is rejected into the LTR. After the rejection of |QL|, the heat engine’s state will resume to the initial state i. ...
... by the amount |QH| from HTR, turning part of this heat into work |W|, and the balance of heat, |QL| =|QH| -|W|, is rejected into the LTR. After the rejection of |QL|, the heat engine’s state will resume to the initial state i. ...
Basic Properties of the Atmosphere
... • The Arctic Ocean has a large amount of heat (because of large mass) even though the temperature is low. • Air in an oven at 500 F has high temperature but little heat. • However, touch anything solid in the oven, and you’ll get burned. Same temperature, much larger amount of heat. 1. Heat, Tempera ...
... • The Arctic Ocean has a large amount of heat (because of large mass) even though the temperature is low. • Air in an oven at 500 F has high temperature but little heat. • However, touch anything solid in the oven, and you’ll get burned. Same temperature, much larger amount of heat. 1. Heat, Tempera ...
Basic Properties of the Atmosphere
... • The Arctic Ocean has a large amount of heat (because of large mass) even though the temperature is low. • Air in an oven at 500 F has high temperature but little heat. • However, touch anything solid in the oven, and you’ll get burned. Same temperature, much larger amount of heat. 1. Heat, Tempera ...
... • The Arctic Ocean has a large amount of heat (because of large mass) even though the temperature is low. • Air in an oven at 500 F has high temperature but little heat. • However, touch anything solid in the oven, and you’ll get burned. Same temperature, much larger amount of heat. 1. Heat, Tempera ...
Ideal gas
... the pressure of a mixture of gases simply is the sum of the partial pressures of the individual components. ...
... the pressure of a mixture of gases simply is the sum of the partial pressures of the individual components. ...