Atmospheric circulation
... Why are clouds in lows? • Warm air can hold more water vapor than cool air ...
... Why are clouds in lows? • Warm air can hold more water vapor than cool air ...
Kinetic Molecular Theory
... -Heat energy is transferred through the COLLISION OF MOLECULES. Examples: Metals are good conductors. Glass is an insulator. -Convection -Heat is transferred through convection currents. This occurs when FLUIDS RISE AND FALL THROUGH EXPANSION AND CONTRACTION. Examples: In the earth (continents move, ...
... -Heat energy is transferred through the COLLISION OF MOLECULES. Examples: Metals are good conductors. Glass is an insulator. -Convection -Heat is transferred through convection currents. This occurs when FLUIDS RISE AND FALL THROUGH EXPANSION AND CONTRACTION. Examples: In the earth (continents move, ...
Atmospheric circulation
... the apparent motion is to the right. [An object moving north from the equator has an initial eastward velocity. In NH objects moving south have the land rotate faster under them.] the apparent motion is to the left. ...
... the apparent motion is to the right. [An object moving north from the equator has an initial eastward velocity. In NH objects moving south have the land rotate faster under them.] the apparent motion is to the left. ...
Chapter Two Atoms & The Periodic Table
... less stable and more reactive Lower energetic substances are typically more stable and less reactive ...
... less stable and more reactive Lower energetic substances are typically more stable and less reactive ...
Word
... 3. Keeping warm or cool Hot water bottles and ice-packs rely on the high heat capacity of water to keep other items hot or cold. The water can lose or gain a lot of energy while changing temperature very little compared to most other substances. Other materials, such as wheat, are also used to fill ...
... 3. Keeping warm or cool Hot water bottles and ice-packs rely on the high heat capacity of water to keep other items hot or cold. The water can lose or gain a lot of energy while changing temperature very little compared to most other substances. Other materials, such as wheat, are also used to fill ...
Weather Dynamics
... equally by climate change. • Low-lying and coastal areas face the risks associated with rising sea levels. • Scientists have also determined that warming will be greater in polar regions than nearer to the equator, and that continental interiors will experience greater warming than coastal areas. ...
... equally by climate change. • Low-lying and coastal areas face the risks associated with rising sea levels. • Scientists have also determined that warming will be greater in polar regions than nearer to the equator, and that continental interiors will experience greater warming than coastal areas. ...
ASLab_100Specific Heat Inquiry
... One characteristic or property of all solids and liquids is something called the Specific Heat, abbreviated as Cv. This quantity represents the amount of heat required to raise or lower a given quantity (a gram or a Kilogram) by one degree. Water has an extremely high Specific Heat (1 calorie per gr ...
... One characteristic or property of all solids and liquids is something called the Specific Heat, abbreviated as Cv. This quantity represents the amount of heat required to raise or lower a given quantity (a gram or a Kilogram) by one degree. Water has an extremely high Specific Heat (1 calorie per gr ...
Process Heat Transfer Lab - University of Engineering and Technology
... The bench-top cooling tower is necessary to study and demonstrate the industrial practice in which a cooling tower is used to cool water from a process. Students can quickly investigate the effects of air flow rate, water flow rate, water temperature, cooling load, air temperature and humidity at in ...
... The bench-top cooling tower is necessary to study and demonstrate the industrial practice in which a cooling tower is used to cool water from a process. Students can quickly investigate the effects of air flow rate, water flow rate, water temperature, cooling load, air temperature and humidity at in ...
Chapters 19&20
... • Quantity Q + W = ΔEint (change of internal energy) is path-independent • 1st law of thermodynamics: the internal energy of a system increases if heat is added to the system or work is done on the system ...
... • Quantity Q + W = ΔEint (change of internal energy) is path-independent • 1st law of thermodynamics: the internal energy of a system increases if heat is added to the system or work is done on the system ...
heat engine
... True or false: Which one is better? A gas hot water heater or a solar water heater? ...
... True or false: Which one is better? A gas hot water heater or a solar water heater? ...
Unit 8-10 Review Answers
... (a) Molecules in a liquid are much more closely packed than molecules in a gas. (b) Molecules in a liquid can vibrate and rotate, but they cannot move about freely as molecules in a gas. (c) Liquids are much more difficult to compress into a smaller volume than are gases. (d) Liquids diffuse more sl ...
... (a) Molecules in a liquid are much more closely packed than molecules in a gas. (b) Molecules in a liquid can vibrate and rotate, but they cannot move about freely as molecules in a gas. (c) Liquids are much more difficult to compress into a smaller volume than are gases. (d) Liquids diffuse more sl ...
STATES OF MATTER
... comet might look like. This is a picture of dry ice (frozen CO2) sublimating. ...
... comet might look like. This is a picture of dry ice (frozen CO2) sublimating. ...
Temperature and Heat
... accommodate thermal expansion. (Reproduced by permission of JLM Visuals) ...
... accommodate thermal expansion. (Reproduced by permission of JLM Visuals) ...
Temperature and Heat Temperature Depends on Particle Movement
... • Heat is measured by the units of calorie and joule (J). • calorie: The amount of energy needed to raise the temperature of 1 gram of water by 1oC ...
... • Heat is measured by the units of calorie and joule (J). • calorie: The amount of energy needed to raise the temperature of 1 gram of water by 1oC ...
Electrical Equivalent of Heat
... supply. The final form of energy is heat as it radiates outward from and throughout the wire. The amount of electrical energy transformed into heat will depend on the current passing through the wire, the number and speed of the electrons and the resistance in the wire which is related to the above ...
... supply. The final form of energy is heat as it radiates outward from and throughout the wire. The amount of electrical energy transformed into heat will depend on the current passing through the wire, the number and speed of the electrons and the resistance in the wire which is related to the above ...
Heat and the Umpire
... come out of the game or be seen as weak. Its not weakness, its being human and we all are vulnerable. Look for mental status or behavior changes among your colleagues because they won’t recognize this themselves. Often the skin is dry instead of moist and the skin may turn very flushed or red. Breat ...
... come out of the game or be seen as weak. Its not weakness, its being human and we all are vulnerable. Look for mental status or behavior changes among your colleagues because they won’t recognize this themselves. Often the skin is dry instead of moist and the skin may turn very flushed or red. Breat ...
Joule`s Law and Heat Transfer Name:
... 7. Open DataStudio, select "Open Activity", select "Library", select "Physics Labs folder", and select "P16-Temperature and Heat". Click on the digits display and click start. 8. Plug in the power, and stir the water gently with the temperature sensor. 9. When the temperature reaches 20oC, the PC wi ...
... 7. Open DataStudio, select "Open Activity", select "Library", select "Physics Labs folder", and select "P16-Temperature and Heat". Click on the digits display and click start. 8. Plug in the power, and stir the water gently with the temperature sensor. 9. When the temperature reaches 20oC, the PC wi ...
Heat and Temperature
... Atoms can move into a state from which it is impossible to extract more energy—this is Absolute Zero ...
... Atoms can move into a state from which it is impossible to extract more energy—this is Absolute Zero ...
Chem 1010 Tutorials Tutorial 9A – Heat and Work Fall 2013
... In order to raise the temperature of a particular pond by 2.3 K, 5.2 x 1028 kJ of heat are required. a) What is the mass of the pond? (specific heat of water is 4.184 J·g–1·°C –1) b) What is the heat capacity of the pond? c) How much heat would be given off if the temperature of the pond decreased b ...
... In order to raise the temperature of a particular pond by 2.3 K, 5.2 x 1028 kJ of heat are required. a) What is the mass of the pond? (specific heat of water is 4.184 J·g–1·°C –1) b) What is the heat capacity of the pond? c) How much heat would be given off if the temperature of the pond decreased b ...
Thermochemistry
... • Heat (q) - energy that is transferred from one object to another because of a temperature difference between them. • Heat always flows from a warmer object to a cooler object. • Heat cannot be measured directly. ...
... • Heat (q) - energy that is transferred from one object to another because of a temperature difference between them. • Heat always flows from a warmer object to a cooler object. • Heat cannot be measured directly. ...
Phy213_2 - Personal.psu.edu
... equilibrium with a third body T, then they are in thermal equilibrium with each other. Thermal equilibrium: two objects in thermal contact cease to have any exchange of heat. Thermal contact : Heat can be exchanged. Heat: energy exchanged between objects due to their temperature difference. Temperat ...
... equilibrium with a third body T, then they are in thermal equilibrium with each other. Thermal equilibrium: two objects in thermal contact cease to have any exchange of heat. Thermal contact : Heat can be exchanged. Heat: energy exchanged between objects due to their temperature difference. Temperat ...
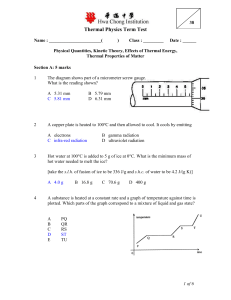
Physical Quantities, Kinetic Theory, Effects of
... Hot water at 100°C is added to 5 g of ice at 0°C. What is the minimum mass of hot water needed to melt the ice? [take the s.l.h. of fusion of ice to be 336 J/g and s.h.c. of water to be 4.2 J/(g K)] ...
... Hot water at 100°C is added to 5 g of ice at 0°C. What is the minimum mass of hot water needed to melt the ice? [take the s.l.h. of fusion of ice to be 336 J/g and s.h.c. of water to be 4.2 J/(g K)] ...
Energy 1
... • An internal combustion engine burns fuel inside the engine in chambers or cylinders. They only convert around 26% of chemicla energy to mechanical energy. Not efficient machines = pollution. ...
... • An internal combustion engine burns fuel inside the engine in chambers or cylinders. They only convert around 26% of chemicla energy to mechanical energy. Not efficient machines = pollution. ...
11-Heat Energy
... Heat Capacity Heat Capacity is a measure of how much heat energy must be added to raise the temperature of an object. A large heat capacity means that a lot of heat must be added transferred to raise the temperature of the object by a given amount. A bigger object of the same material has a bigger ...
... Heat Capacity Heat Capacity is a measure of how much heat energy must be added to raise the temperature of an object. A large heat capacity means that a lot of heat must be added transferred to raise the temperature of the object by a given amount. A bigger object of the same material has a bigger ...
10 Temperature and Heat
... Yes. The change in volume will indicate a change in temperature, according to the “predictable way”. No. This is not reliable since it is really a comparison between our skin temperature and the skin temperature of the other person. We are assuming that our skin temperature is that of a person with ...
... Yes. The change in volume will indicate a change in temperature, according to the “predictable way”. No. This is not reliable since it is really a comparison between our skin temperature and the skin temperature of the other person. We are assuming that our skin temperature is that of a person with ...