Specific Heat!
... changes from a temperature of 20.0°C to 27.4ºC. How much heat energy did it gain? A 200 J ...
... changes from a temperature of 20.0°C to 27.4ºC. How much heat energy did it gain? A 200 J ...
Thermodynamics
... First: the conservation of energy - internal energy of a system. Second: why things happen as they do - entropy of a system Third: Absolute Zero is unattainable, negative temperatures ...
... First: the conservation of energy - internal energy of a system. Second: why things happen as they do - entropy of a system Third: Absolute Zero is unattainable, negative temperatures ...
Joule`s Law and Heat Transfer Name:
... Theory: We will use electrical energy to heat a certain amount of cold-water. Electrical energy is measured in Joules and heat is measured in calories. In this activity we will look at a version of Joule's experiment, which gives a relationship between calorie and Joule. To do this we need to measur ...
... Theory: We will use electrical energy to heat a certain amount of cold-water. Electrical energy is measured in Joules and heat is measured in calories. In this activity we will look at a version of Joule's experiment, which gives a relationship between calorie and Joule. To do this we need to measur ...
Calorimetry worksheet - MRS. STOTTS CHEMISTRY
... There are several terms used in this chapter that sound very similar. Use the data provided to calculate each of them to clarify the differences. I’ve added some “Notes” that I hope will help. 74.8 J of heat is required to raise the temperature of 18.69 g of silver from 10.0C to 27.0C. a. What is ...
... There are several terms used in this chapter that sound very similar. Use the data provided to calculate each of them to clarify the differences. I’ve added some “Notes” that I hope will help. 74.8 J of heat is required to raise the temperature of 18.69 g of silver from 10.0C to 27.0C. a. What is ...
Chapter 6 Lesson 2 Name_____________ Describe the three ways
... In Figure 6-1, thermal energy is transferred to the sunbather in room B primarily by ____________________. In Figure 6-1, most of the heat provided by the fireplace in room C goes up the chimney and is therefore transferred by ____________________. In Figure 6-1, the thermal energy of the iron in ro ...
... In Figure 6-1, thermal energy is transferred to the sunbather in room B primarily by ____________________. In Figure 6-1, most of the heat provided by the fireplace in room C goes up the chimney and is therefore transferred by ____________________. In Figure 6-1, the thermal energy of the iron in ro ...
Energy Study Guide
... Read over your notes, and rework your homework assignments and quizzes (especially those you didn’t do well on). You are responsible for knowing W=F·d, but you will be given Q=m·T·Cp and the necessary specific heat values. You will do AWESOME on this test if you can do the following things. ...
... Read over your notes, and rework your homework assignments and quizzes (especially those you didn’t do well on). You are responsible for knowing W=F·d, but you will be given Q=m·T·Cp and the necessary specific heat values. You will do AWESOME on this test if you can do the following things. ...
Heat, Temperature and Atmospheric Circulations
... different parts may be different temperatures ...
... different parts may be different temperatures ...
Review for test number 2. Know the definitions of the different types
... Be able to convert from Celsius to Fahrenheit: TF = (9/5)TC + 32 ...
... Be able to convert from Celsius to Fahrenheit: TF = (9/5)TC + 32 ...
53 - Angelfire
... 20.32. A sample of an ideal gas goes through the process shown in Figure P20.32. From A to B, the process is adiabatic; from B to C, it is isobaric, with 100 kJ of energy entering the system by heat. From C to D, the process is isothermal; from D to A, it is isobaric, with 150 kJ of energy leaving t ...
... 20.32. A sample of an ideal gas goes through the process shown in Figure P20.32. From A to B, the process is adiabatic; from B to C, it is isobaric, with 100 kJ of energy entering the system by heat. From C to D, the process is isothermal; from D to A, it is isobaric, with 150 kJ of energy leaving t ...
NSTA Meteorology Reading 5 • Weather and the Redistribution of
... ‣ Weather = a response to the unequal heating of Earth’s atmosphere ‣ Temperature gradients created by imbalances in rates of heating and cooling from once place to another within the atmosphere ‣ Many different kinds of energy ‣ Energy can be transformed from one type to another ‣ The total amount ...
... ‣ Weather = a response to the unequal heating of Earth’s atmosphere ‣ Temperature gradients created by imbalances in rates of heating and cooling from once place to another within the atmosphere ‣ Many different kinds of energy ‣ Energy can be transformed from one type to another ‣ The total amount ...
Heat Transfer LAB
... the flow of heat or electricity easily. Convection is the transfer of heat by currents within a fluid; liquid or gas. The hotter the fluid the faster the particle movement and the less dense the material is thus making it rise. The cooler a fluid is the slower the particle movement and the more dens ...
... the flow of heat or electricity easily. Convection is the transfer of heat by currents within a fluid; liquid or gas. The hotter the fluid the faster the particle movement and the less dense the material is thus making it rise. The cooler a fluid is the slower the particle movement and the more dens ...
The Specific Heat Capacity of Metals
... it to boil for about five minutes so that the metal reaches the temperature of the boiling water. Take the temperature of the water. Assume this is also the temperature of the metal. Record this temperature in the table. 3. Add 100 g of cold water to an insulated cup. Quickly remove the metal sample ...
... it to boil for about five minutes so that the metal reaches the temperature of the boiling water. Take the temperature of the water. Assume this is also the temperature of the metal. Record this temperature in the table. 3. Add 100 g of cold water to an insulated cup. Quickly remove the metal sample ...
Thermodynamics
... energy of a ball when we drop it on the floor? Answer: It goes into heat energy. Question: What is heat energy? ...
... energy of a ball when we drop it on the floor? Answer: It goes into heat energy. Question: What is heat energy? ...
ENVIRONMENT & ANIMAL HEALTH
... • Farm animals and humans are classified as homeotherms since they maintain a constant core body temperature across a wide range of environments. • Poikilotherms (cold blooded animals such as fish and snakes) do not maintain a constant body temperature, but rather are influenced by the environment. ...
... • Farm animals and humans are classified as homeotherms since they maintain a constant core body temperature across a wide range of environments. • Poikilotherms (cold blooded animals such as fish and snakes) do not maintain a constant body temperature, but rather are influenced by the environment. ...
Recitation 3.2 Temperature/Heat
... the tube has a thin aluminum plate with a mass of 1.57g. The specific heat of aluminum is 0.90 J/g-K. The tube is 0.90 m long. Turn the tube over so the BB’s fall on the instrumented aluminum plate five times. Measure the temperature change of the aluminum plate. Compare the mechanical energy to the ...
... the tube has a thin aluminum plate with a mass of 1.57g. The specific heat of aluminum is 0.90 J/g-K. The tube is 0.90 m long. Turn the tube over so the BB’s fall on the instrumented aluminum plate five times. Measure the temperature change of the aluminum plate. Compare the mechanical energy to the ...
Chapter Two Atoms & The Periodic Table
... Bclosed system (heat only pass through) Cisolated system (no heat or mass transfer) ...
... Bclosed system (heat only pass through) Cisolated system (no heat or mass transfer) ...
Temperature, Heat, and Expansion
... Metals are the best conductors. If you touch a piece of metal and a piece of wood that have been outside, which will feel colder? Which is really colder? ...
... Metals are the best conductors. If you touch a piece of metal and a piece of wood that have been outside, which will feel colder? Which is really colder? ...
DriTherm®: Brick Cavity Wall Insulation
... warm reducing heat transfer from inside the home to the outside. ...
... warm reducing heat transfer from inside the home to the outside. ...
Cases – Chapter 8 1. A solar panel is an electronic device that
... disease,” a painful or even fatal disorder in which gas bubbles formed inside their tissues. Because they bent over in pain, their illness was called “the bends.” a. The workers would enter the caisson while it was at atmospheric pressure. The doors were then sealed and air was pumped in. The tempe ...
... disease,” a painful or even fatal disorder in which gas bubbles formed inside their tissues. Because they bent over in pain, their illness was called “the bends.” a. The workers would enter the caisson while it was at atmospheric pressure. The doors were then sealed and air was pumped in. The tempe ...
Waste Heat Recovery from PV Panels FINAL PRESENTATION
... • After observation of the tank temperature at the end of each testing session, it was determined that 3 units alone would not be sufficient to provide enough heat for domestic hot water usage. • Temperature to safely kill off formation of Legionella bacteria, source of Legionnaire’s disease, is 140 ...
... • After observation of the tank temperature at the end of each testing session, it was determined that 3 units alone would not be sufficient to provide enough heat for domestic hot water usage. • Temperature to safely kill off formation of Legionella bacteria, source of Legionnaire’s disease, is 140 ...
Document
... 15. How much heat energy is required to heat a 14.75 g sample of ice at -23˚C to steam at 121˚C? Cice = 2.06 J/g˚C Csteam = 2.02 J/g˚C ΔHfus = 6.02 kJ/mol ΔHvap = 40.7 kJ/mol ...
... 15. How much heat energy is required to heat a 14.75 g sample of ice at -23˚C to steam at 121˚C? Cice = 2.06 J/g˚C Csteam = 2.02 J/g˚C ΔHfus = 6.02 kJ/mol ΔHvap = 40.7 kJ/mol ...
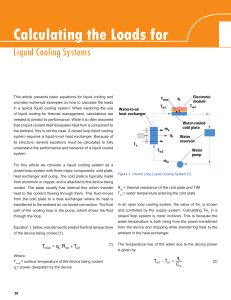
Calculating the Loads for Liquid cooling Systems
... This article presents basic equations for liquid cooling and provides numerical examples on how to calculate the loads in a typical liquid cooling system. When exploring the use of liquid cooling for thermal management, calculations are needed to predict its performance. While it is often assumed th ...
... This article presents basic equations for liquid cooling and provides numerical examples on how to calculate the loads in a typical liquid cooling system. When exploring the use of liquid cooling for thermal management, calculations are needed to predict its performance. While it is often assumed th ...
Heat illness
... What to Do to Keep Yourself Cool During normal work activities, especially, during summer months, workers may be required to work in hot environments. When the body is unable to cool itself by sweating, several heat-induced illnesses can occur, and can result in death. ...
... What to Do to Keep Yourself Cool During normal work activities, especially, during summer months, workers may be required to work in hot environments. When the body is unable to cool itself by sweating, several heat-induced illnesses can occur, and can result in death. ...
Heat Transfer in the Atmosphere
... Two different materials at the same temperature have different emissivities. Which one glows the brightest? ...
... Two different materials at the same temperature have different emissivities. Which one glows the brightest? ...