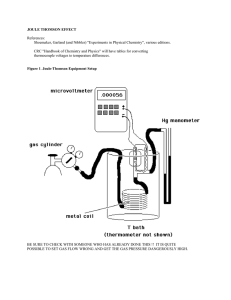
joule thomson effect
... Read the Shoemaker, et al. source for a more general background. The thermocouples are both copper-constantan, and you already have a calibration equation from lab 1 which can be used to convert voltage differences between the two junctions to temperature differences. The microvoltmeter is very sens ...
... Read the Shoemaker, et al. source for a more general background. The thermocouples are both copper-constantan, and you already have a calibration equation from lab 1 which can be used to convert voltage differences between the two junctions to temperature differences. The microvoltmeter is very sens ...
Heat And Thermodynamics - Figure B
... are placed into thermal contact. It can flow from high temperature to low temperature till temperature of the two bodies becomes same. Thus, we can say that heat is the energy in transit. Heat is not property of system, a system can give out heat or can absorb heat but it does not contain heat. Th ...
... are placed into thermal contact. It can flow from high temperature to low temperature till temperature of the two bodies becomes same. Thus, we can say that heat is the energy in transit. Heat is not property of system, a system can give out heat or can absorb heat but it does not contain heat. Th ...
f21/2509/2009 githua scolastica njoki heat and mass transfer
... Derive an expression for the critical radius of insulation for a radial system Adding insulation to a cylindrical piece or a spherical shell increases the conduction resistance of the insulation layer but decreases the convection resistance of the surface because of the increase in the outer surface ...
... Derive an expression for the critical radius of insulation for a radial system Adding insulation to a cylindrical piece or a spherical shell increases the conduction resistance of the insulation layer but decreases the convection resistance of the surface because of the increase in the outer surface ...
PS#3
... 35.46 J K-1 mol-1. Ethylene: Cp,m = 42.17 J K-1 mol-1. Hint: Think about H for the overall process. 2. Considering H2O to be a rigid nonlinear molecule, what value of Cp,m would be expected classically, if we take into account translation and rotation, but not vibration? If translation, rotation, a ...
... 35.46 J K-1 mol-1. Ethylene: Cp,m = 42.17 J K-1 mol-1. Hint: Think about H for the overall process. 2. Considering H2O to be a rigid nonlinear molecule, what value of Cp,m would be expected classically, if we take into account translation and rotation, but not vibration? If translation, rotation, a ...
Transfer of Thermal Energy worksheet - dubai
... If you have stood in front of a fireplace or near a campfire, you have felt the heat transfer known as radiation. The side of you nearest the fire warms, while your other side remains unaffected by the heat. Although you are surrounded by air, the air has nothing to do with this transfer of heat. He ...
... If you have stood in front of a fireplace or near a campfire, you have felt the heat transfer known as radiation. The side of you nearest the fire warms, while your other side remains unaffected by the heat. Although you are surrounded by air, the air has nothing to do with this transfer of heat. He ...
Heat - Geography1000
... the energy passes through a longer atmosphere (larger angle) red, orange, and yellow left. ...
... the energy passes through a longer atmosphere (larger angle) red, orange, and yellow left. ...
Solids, Liquids, and Gases Review
... 1. Adding thermal energy to a solid normally causes a temperature change in the solid. What happens to the thermal energy and the temperature as the solid melts? ...
... 1. Adding thermal energy to a solid normally causes a temperature change in the solid. What happens to the thermal energy and the temperature as the solid melts? ...
Study guide answers ch 5
... Kinetic energy is energy of motion and potential energy is stored energy. They convert back and forth due to the law of conservation of energy. ...
... Kinetic energy is energy of motion and potential energy is stored energy. They convert back and forth due to the law of conservation of energy. ...
Heat Transfer Oil
... In many industrial applications heating is provided indirectly by circulating hot oil through a heat exchanger, thus reducing hot spots and increasing the safety of the heating process. In quenching applications, heat is required to be rapidly drawn away from the parts in contact with the oil. Due t ...
... In many industrial applications heating is provided indirectly by circulating hot oil through a heat exchanger, thus reducing hot spots and increasing the safety of the heating process. In quenching applications, heat is required to be rapidly drawn away from the parts in contact with the oil. Due t ...
chapter 3 new blank
... • Change of state from _______________________ • Ex: Ice to liquid water • Melting point:________________ at which substance changes from solid to liquid • Different for different substances • Heat is absorbed Is ____________ (endo= into) (them=heat) • Heat energy causes the particles to increase mo ...
... • Change of state from _______________________ • Ex: Ice to liquid water • Melting point:________________ at which substance changes from solid to liquid • Different for different substances • Heat is absorbed Is ____________ (endo= into) (them=heat) • Heat energy causes the particles to increase mo ...
Chapter 2 Study Guide
... 2. Give examples of each type of heat transfer. 3. Explain why warm air rises and cool air sinks. 4. What type of heat transfer causes most of the heating in the troposphere? Section 3: Winds ...
... 2. Give examples of each type of heat transfer. 3. Explain why warm air rises and cool air sinks. 4. What type of heat transfer causes most of the heating in the troposphere? Section 3: Winds ...
1-14 The filament of a 150 W incandescent lamp is 5 cm long and
... 1-14 The filament of a 150 W incandescent lamp is 5 cm long and has a diameter of 0.5 mm. The heat flux on the surface of the filament, the heat flux on the surface of the glass bulb, and the annual electricity cost of the bulb are to be determined. Assumptions Heat transfer from the surface of the ...
... 1-14 The filament of a 150 W incandescent lamp is 5 cm long and has a diameter of 0.5 mm. The heat flux on the surface of the filament, the heat flux on the surface of the glass bulb, and the annual electricity cost of the bulb are to be determined. Assumptions Heat transfer from the surface of the ...
15 Stout St
... 2 No. smoke clearance supply fans. These fans shall be installed in parallel to the main air handling units and utilize the same supply riser duct. ...
... 2 No. smoke clearance supply fans. These fans shall be installed in parallel to the main air handling units and utilize the same supply riser duct. ...
how to wire electric heat relays - Grover Electric and Plumbing Supply
... therm o sta ts; how ever i t is e xtremely difficult to adjust them so that the temperature throughout the area remains even. Two thermostats in one room tend to burden one heat source with more than its share of the work load. In areas requiring over 5,500 watts of heat, it is almo st always necess ...
... therm o sta ts; how ever i t is e xtremely difficult to adjust them so that the temperature throughout the area remains even. Two thermostats in one room tend to burden one heat source with more than its share of the work load. In areas requiring over 5,500 watts of heat, it is almo st always necess ...
INTRODUCTION UNIT TEST #2 (3085) Multiple Choice: Choose the
... A. The molecules become larger, spread out and have more kinetic energy. B. The molecules become smaller, contract and have less kinetic energy. C. The molecules become faster, spread out and have more kinetic energy. D. The molecules become slower, contract and have less kinetic energy. 58. Which s ...
... A. The molecules become larger, spread out and have more kinetic energy. B. The molecules become smaller, contract and have less kinetic energy. C. The molecules become faster, spread out and have more kinetic energy. D. The molecules become slower, contract and have less kinetic energy. 58. Which s ...
Thermodynamics
... an initial temperature of 25.0°C. As a result of the reaction, the temperature of the water changes to 31.0°C. The heat flow is calculated: qwater = 4.18 J/(g·°C) x 200 g x (31.0°C - 25.0°C) qwater = +5.0 x 103 J ...
... an initial temperature of 25.0°C. As a result of the reaction, the temperature of the water changes to 31.0°C. The heat flow is calculated: qwater = 4.18 J/(g·°C) x 200 g x (31.0°C - 25.0°C) qwater = +5.0 x 103 J ...
Allow the sun`s warm rays in and heat up energy
... STAR® requirements in the Northern U.S. Zone and, by flipping the IG unit and orienting the coating to the #2 surface, meeting requirements for the North-Central Zone. ...
... STAR® requirements in the Northern U.S. Zone and, by flipping the IG unit and orienting the coating to the #2 surface, meeting requirements for the North-Central Zone. ...
Heat - Warren County Schools
... apart they move. This spreading out of atoms happens in solids, liquids and gases. The term for this type of expansion is called THERMAL ...
... apart they move. This spreading out of atoms happens in solids, liquids and gases. The term for this type of expansion is called THERMAL ...
heat exchanger - Universitas Mercu Buana
... The basic design of a heat exchanger normally has two fluids of different temperatures separated by some conducting medium. The most common design has one fluid flowing through metal tubes and the other fluid flowing around the tubes. On either side of the tube, heat is transferred by convection. He ...
... The basic design of a heat exchanger normally has two fluids of different temperatures separated by some conducting medium. The most common design has one fluid flowing through metal tubes and the other fluid flowing around the tubes. On either side of the tube, heat is transferred by convection. He ...
Too Hot to Handle, Too Cold to Hold
... 2) For space/water heating, we have an excess of waste heat from power plants and an abundance of geothermal heat. 3) To fill in gaps and further reduce costs, integrated renewables (wind/solar) are a cure-all. ...
... 2) For space/water heating, we have an excess of waste heat from power plants and an abundance of geothermal heat. 3) To fill in gaps and further reduce costs, integrated renewables (wind/solar) are a cure-all. ...
Name: Date: ______ Thermochemistry Round Robin
... 15. How much heat energy is required to heat a 14.75 g sample of ice at -23˚C to steam at 121˚C? Cice = 2.06 J/g˚C Csteam = 2.02 J/g˚C ΔHfus = 6.02 kJ/mol ΔHvap = 40.7 kJ/mol ...
... 15. How much heat energy is required to heat a 14.75 g sample of ice at -23˚C to steam at 121˚C? Cice = 2.06 J/g˚C Csteam = 2.02 J/g˚C ΔHfus = 6.02 kJ/mol ΔHvap = 40.7 kJ/mol ...
Abstract
... solar furnace in the James. S. Markiewicz Solar Energy Research Facility in order to substitute solar thermal energy for electric energy. A major challenge of the research effort is to develop an interface that allows the integration of the solar thermal energy into the electrolytic cell where the M ...
... solar furnace in the James. S. Markiewicz Solar Energy Research Facility in order to substitute solar thermal energy for electric energy. A major challenge of the research effort is to develop an interface that allows the integration of the solar thermal energy into the electrolytic cell where the M ...
L 17 - Thermodynamics [2] Thermal Expansion Coefficients of linear
... Heat transfer by Convection • heat is transferred from one location to another by the bulk movement and mixing of liquids or gases (fluids), but NOT in solids. • when water is boiled, hot water at the bottom rises and mixes with cooler water at the top • Hot air rises: • want heated air into lower ...
... Heat transfer by Convection • heat is transferred from one location to another by the bulk movement and mixing of liquids or gases (fluids), but NOT in solids. • when water is boiled, hot water at the bottom rises and mixes with cooler water at the top • Hot air rises: • want heated air into lower ...























![L 17 - Thermodynamics [2] Thermal Expansion Coefficients of linear](http://s1.studyres.com/store/data/014728078_1-e88e92f3857e030978e2ede6a9072797-300x300.png)