Thermo Equilibrium Essay - Dyno Guzman`s Digital Portfolio
... goes or where S is the entropy of the system. The entropy is generally related to the heat term by dS = δQ / T ...
... goes or where S is the entropy of the system. The entropy is generally related to the heat term by dS = δQ / T ...
Section - I: SHORT DESCRIPTIVE QUESTIONS Marks: 10 x 1 = 10
... 90% of capacity, meeting the entire power requirement of the plant, which is presently drawn from grid supply. The co-gen plant will also meet the steam requirement of 10 TPH, which is presently generated in a gas fired boiler with 86% efficiency on N.C.V. basis. Calculate the differential cost betw ...
... 90% of capacity, meeting the entire power requirement of the plant, which is presently drawn from grid supply. The co-gen plant will also meet the steam requirement of 10 TPH, which is presently generated in a gas fired boiler with 86% efficiency on N.C.V. basis. Calculate the differential cost betw ...
Thermochemistry PPT
... • Thermochemistry – study of energy changes that occur during phase changes and chem. rxns. • Chem. Potential Energy – energy stored in chemical bonds. ...
... • Thermochemistry – study of energy changes that occur during phase changes and chem. rxns. • Chem. Potential Energy – energy stored in chemical bonds. ...
Chapter 8 Those incredible water molecules Water is the most
... surface of a liquid to go into the liquid. The surface tension also accounts for the spherical shape of liquid drops, especially in the weightless environment. ...
... surface of a liquid to go into the liquid. The surface tension also accounts for the spherical shape of liquid drops, especially in the weightless environment. ...
Intro, KM Theory, Temp Conversion, Heat transfer Thermodynamics
... Heat – The energy transferred between objects because of the temperature difference. Because heat is energy we can measure it in Joules. Melting Point –The temperature at which a solid object becomes a liquid. Boiling Point – The temperature at which a liquid object becomes a gas. Heat of fusion – T ...
... Heat – The energy transferred between objects because of the temperature difference. Because heat is energy we can measure it in Joules. Melting Point –The temperature at which a solid object becomes a liquid. Boiling Point – The temperature at which a liquid object becomes a gas. Heat of fusion – T ...
Temperature
... Do all objects absorb and store heat in the same way? If both cars are left in the sun in the senior lot for the same amount of time will the interiors be at the same temperature at lunch ...
... Do all objects absorb and store heat in the same way? If both cars are left in the sun in the senior lot for the same amount of time will the interiors be at the same temperature at lunch ...
B3_Energy_transfers
... • Appreciate that raising the temperature of one kilogram of different materials requires the supply of different quantities of energy and appreciate some of the effects of materials having different specific heat capacities. • Use the equation: change in internal energy (J) = mass (kg) x specific h ...
... • Appreciate that raising the temperature of one kilogram of different materials requires the supply of different quantities of energy and appreciate some of the effects of materials having different specific heat capacities. • Use the equation: change in internal energy (J) = mass (kg) x specific h ...
95HE-4
... 7. A length of steel wire 2.5mm diameter is heated from 13OC to 113OC and its ends are securely fastened while the wire is hot. Find the stress in the wire when it cools down to its original temperature, assuming the elastic limit of the wire is not exceeded. Take the coefficient of linear expansion ...
... 7. A length of steel wire 2.5mm diameter is heated from 13OC to 113OC and its ends are securely fastened while the wire is hot. Find the stress in the wire when it cools down to its original temperature, assuming the elastic limit of the wire is not exceeded. Take the coefficient of linear expansion ...
Thermal Energy FlowData set table 1
... adequately protected with a membrane to allow for irrigation. TPO is manufactured using ethylene propylene rubber, a material that has excellent flexibility as well as durability regardless of the weather type. This material works perfectly regardless of whether the weather is hot, cold or wet. Clay ...
... adequately protected with a membrane to allow for irrigation. TPO is manufactured using ethylene propylene rubber, a material that has excellent flexibility as well as durability regardless of the weather type. This material works perfectly regardless of whether the weather is hot, cold or wet. Clay ...
Thermodynamics Exam 1 Info/Problems
... from the attic to a cold outside than from the interior walls. 24. Ice can obviously be colder than 0 C; if you take an ice cube an put it outside on a cold La Crosse winter day, it will probably become much colder than 0 C. Ice may be able to exist at temperatures above that, but certainly not at ...
... from the attic to a cold outside than from the interior walls. 24. Ice can obviously be colder than 0 C; if you take an ice cube an put it outside on a cold La Crosse winter day, it will probably become much colder than 0 C. Ice may be able to exist at temperatures above that, but certainly not at ...
Name
... Part A: Match the terms on the left with the explanations and situations on the right. Some answers may be used more than once and some may not be used at all. A. method of heat transfer where particles collide 1. Heat flow B. heat flows slowly in this type of material 2. Convection 3. Thermal Energ ...
... Part A: Match the terms on the left with the explanations and situations on the right. Some answers may be used more than once and some may not be used at all. A. method of heat transfer where particles collide 1. Heat flow B. heat flows slowly in this type of material 2. Convection 3. Thermal Energ ...
Chemistry - Scarsdale Schools
... a) Calculate the amount of heat energy involved in the reaction. ...
... a) Calculate the amount of heat energy involved in the reaction. ...
Change of state - Mrs. Coyle`s College Chemistry
... Molar heats of fusion are generally much smaller than molar heats of vaporization (liquid molecules are packed closer together and more energy need to rearrange from a solid to liquid) ...
... Molar heats of fusion are generally much smaller than molar heats of vaporization (liquid molecules are packed closer together and more energy need to rearrange from a solid to liquid) ...
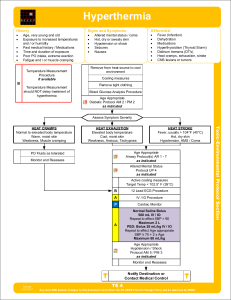
Hyperthermia
... Consists of dehydration, tachycardia, hypotension, temperature 104°F (40°C), and an altered mental status. Sweating generally disappears as body temperature rises above 104°F (40°C). The young and elderly are more prone to be dry with no sweating. Exertional Heat Stroke: In exertional heat stroke (a ...
... Consists of dehydration, tachycardia, hypotension, temperature 104°F (40°C), and an altered mental status. Sweating generally disappears as body temperature rises above 104°F (40°C). The young and elderly are more prone to be dry with no sweating. Exertional Heat Stroke: In exertional heat stroke (a ...
ARCTIC Chills Turbine Power Loss
... WSACs, see “Wet Surface Air Coolers Minimize Water Use by Maximizing Heat Transfer Efficiency” in the September 2008 issue at www.powermag.com.) The total design requirement for makeup cooling water for the ARCTIC system plant is 125 gpm. However, the inlet air chilling is well below the wet bulb te ...
... WSACs, see “Wet Surface Air Coolers Minimize Water Use by Maximizing Heat Transfer Efficiency” in the September 2008 issue at www.powermag.com.) The total design requirement for makeup cooling water for the ARCTIC system plant is 125 gpm. However, the inlet air chilling is well below the wet bulb te ...
THERMAL LABS BOMB CALORIMETER
... The device used to perform this type of experiment is known as a bomb calorimeter (Figure 1). To insure that the bomb and water mass form an adiabatic system a logic unit senses the temperature difference between the mass of water and the water in the surrounding jacket. It controls the flow of hot ...
... The device used to perform this type of experiment is known as a bomb calorimeter (Figure 1). To insure that the bomb and water mass form an adiabatic system a logic unit senses the temperature difference between the mass of water and the water in the surrounding jacket. It controls the flow of hot ...
Calorimetry and Specific Heat
... • How much heat is needed to raise the temperature of 10 grams of water by one degree C? Answer: 10 calories • How much heat is needed to raise the temperature of 10 grams of water by 10 degrees C? • Answer: 100 calories ...
... • How much heat is needed to raise the temperature of 10 grams of water by one degree C? Answer: 10 calories • How much heat is needed to raise the temperature of 10 grams of water by 10 degrees C? • Answer: 100 calories ...
Guided Practice Problems- Exam 3
... the rate of entropy production. Neglect changes in kinetic & potential energy. 17. Air enters a nozzle at 4 bar, 277oC, and 60 m/s and exits at 0.75 bar. Assuming ideal gas behavior, variable specific heats and a reversible and adiabatic process, determine the exit temperature of the air. 18. Air is ...
... the rate of entropy production. Neglect changes in kinetic & potential energy. 17. Air enters a nozzle at 4 bar, 277oC, and 60 m/s and exits at 0.75 bar. Assuming ideal gas behavior, variable specific heats and a reversible and adiabatic process, determine the exit temperature of the air. 18. Air is ...
Chapter 12
... • Thermodynamics- the study of heat transformations into other forms of energy – began with 18th century steam engines ...
... • Thermodynamics- the study of heat transformations into other forms of energy – began with 18th century steam engines ...
Q equations.notebook
... • Where does this energy go? > Particles must overcome forces of attraction to move farther apart during phase change (s → l) ...
... • Where does this energy go? > Particles must overcome forces of attraction to move farther apart during phase change (s → l) ...
Honors Chemistry Quiz Chapter 6: Thermochemistry - Doc-U-Ment
... 1) Energy that is associated with the position or composition of an object is called A) kinetic energy B) thermal energy C) potential energy D) chemical energy E) None of the above. 2) Which of the following signs on q and w represent a system that is doing work on the surroundings, as well as losin ...
... 1) Energy that is associated with the position or composition of an object is called A) kinetic energy B) thermal energy C) potential energy D) chemical energy E) None of the above. 2) Which of the following signs on q and w represent a system that is doing work on the surroundings, as well as losin ...
Snow-melting and Deicing System Using Underground Thermal
... In the heat exchanger, a double-U-shaped pipe system is employed. Compared to conventional a heat exchanger filler, NSC’s original heat exchanger obtains about 20% more geothermal heat. (3) Stable snow-melting performance The use of a low-temperature, phase change material for the latent heat accumu ...
... In the heat exchanger, a double-U-shaped pipe system is employed. Compared to conventional a heat exchanger filler, NSC’s original heat exchanger obtains about 20% more geothermal heat. (3) Stable snow-melting performance The use of a low-temperature, phase change material for the latent heat accumu ...
Name
... a. Which has a higher enthalpy? Products or Reactants b. Was heat absorbed or released? c. Is this an endothermic or exothermic reaction? d. Is ΔH for this reaction positive or negative? e. Would the ΔH be on the left or right side of the yield sign? f. Is the reverse reaction exothermic or endother ...
... a. Which has a higher enthalpy? Products or Reactants b. Was heat absorbed or released? c. Is this an endothermic or exothermic reaction? d. Is ΔH for this reaction positive or negative? e. Would the ΔH be on the left or right side of the yield sign? f. Is the reverse reaction exothermic or endother ...