3. Experimental apparatus
... HPC-5000. Fig. 3.6. shows the temperature measurement system used in the present work. The air bath sample room was a 70-mm diameter steel cylinder with a height of 130 mm. The apparatus was specially designed in the present work. As the sample was explosive material, the amount of the sample should ...
... HPC-5000. Fig. 3.6. shows the temperature measurement system used in the present work. The air bath sample room was a 70-mm diameter steel cylinder with a height of 130 mm. The apparatus was specially designed in the present work. As the sample was explosive material, the amount of the sample should ...
Practice MSL Multiple Choice 1. Compared to the charge and mass
... the same charge and a smaller mass the same charge and the same mass an opposite charge and a smaller mass an opposite charge and the same mass ...
... the same charge and a smaller mass the same charge and the same mass an opposite charge and a smaller mass an opposite charge and the same mass ...
UNIT 2 – Chemical Quantities
... _______________. If you wanted to extract the iron and know how much to expect, you first need to know the _____________________________. Calculate the % composition for ________________. ...
... _______________. If you wanted to extract the iron and know how much to expect, you first need to know the _____________________________. Calculate the % composition for ________________. ...
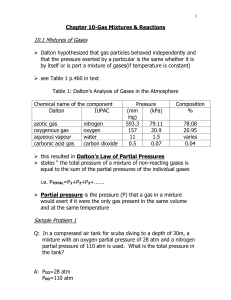
CH 301 Practice Test Questions
... mL flask. At 371 K, the pressure of the vapor in the flask is 754 torr. What is the molar mass in g/mol? 6. What is the density of nitrogen gas at STP? 7. Consider two equal-sized containers, one filled with H2 gas and one with O2 gas at the same temperature and pressure. The average velocity of the ...
... mL flask. At 371 K, the pressure of the vapor in the flask is 754 torr. What is the molar mass in g/mol? 6. What is the density of nitrogen gas at STP? 7. Consider two equal-sized containers, one filled with H2 gas and one with O2 gas at the same temperature and pressure. The average velocity of the ...
Final Exam Review Guide
... Rotation (spinning in place) – all phases Vibration (shaking in place) – all phases Translation (moving from place to place) – Liquid and Gas only Describe the “Kinetic Theory of Gases” and list the three assumptions associated with it. What volume does one mole of any gas occupy at STP? 22.4 L Kine ...
... Rotation (spinning in place) – all phases Vibration (shaking in place) – all phases Translation (moving from place to place) – Liquid and Gas only Describe the “Kinetic Theory of Gases” and list the three assumptions associated with it. What volume does one mole of any gas occupy at STP? 22.4 L Kine ...
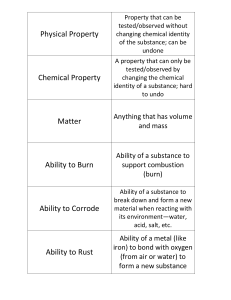
1.2 Properties and Changes of Matter
... The melting points and freezing point are the same for substances ...
... The melting points and freezing point are the same for substances ...
matter - Firelands Local Schools
... The number after and below the atomic symbol indicates the number of that element 1. Example: C16H10N2O2 = 16 carbon, 10 hydrogen, 2 nitrogen, and 2 oxygen ...
... The number after and below the atomic symbol indicates the number of that element 1. Example: C16H10N2O2 = 16 carbon, 10 hydrogen, 2 nitrogen, and 2 oxygen ...
Chapter 6 Notes - Discount Flies
... Evidence for a chemical reaction is sometimes visual, and can include any or all of the following: 1) Color Change 2) Solid Forms 3) Gas Given Off 4) Heat, Cold or Flames ...
... Evidence for a chemical reaction is sometimes visual, and can include any or all of the following: 1) Color Change 2) Solid Forms 3) Gas Given Off 4) Heat, Cold or Flames ...
+ O2 (g)
... 2. states of reactants and products 3. relative numbers of reactant and product molecules ...
... 2. states of reactants and products 3. relative numbers of reactant and product molecules ...
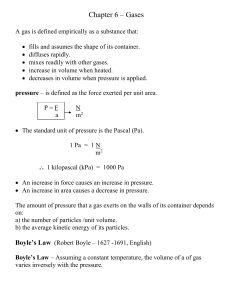
Review Worksheet
... b) Ideal gases consist of small particles (molecules or atoms) that are far apart in comparison to their own size. The molecules of a gas are very __________ compared to the distances between them. c) These particles are considered to be dimensionless points which occupy zero volume. The volume of r ...
... b) Ideal gases consist of small particles (molecules or atoms) that are far apart in comparison to their own size. The molecules of a gas are very __________ compared to the distances between them. c) These particles are considered to be dimensionless points which occupy zero volume. The volume of r ...
CST Review Part 2
... pressure, temperature, and volume of any amount of an ideal gas or any mixture of ideal gases. d. Students know the values and meanings of standard temperature and pressure (STP). e. Students know how to convert between the Celsius and Kelvin temperature scales. f. Students know there is no temperat ...
... pressure, temperature, and volume of any amount of an ideal gas or any mixture of ideal gases. d. Students know the values and meanings of standard temperature and pressure (STP). e. Students know how to convert between the Celsius and Kelvin temperature scales. f. Students know there is no temperat ...
File
... • It is still possible for there to be only one of these elements, if it is bonded to something else. ▫ Example: MgO ...
... • It is still possible for there to be only one of these elements, if it is bonded to something else. ▫ Example: MgO ...
Chapter 6 notes 2015
... If you have two gases at the same temperature and pressure, than : the average kinetic energy of the two gases is the same. (same temp.) ... if there is any difference in the pressure exerted by the two gases, it would be due to the number of particles. however, since we have already stated th ...
... If you have two gases at the same temperature and pressure, than : the average kinetic energy of the two gases is the same. (same temp.) ... if there is any difference in the pressure exerted by the two gases, it would be due to the number of particles. however, since we have already stated th ...
states of matter - Haiku for Ignatius
... Gas to liquid Condensation as it condenses. Heat goes into the Solid to gas Sublimation solid as it sublimates. ...
... Gas to liquid Condensation as it condenses. Heat goes into the Solid to gas Sublimation solid as it sublimates. ...
Study Guide – Unit Test (9-27-13)
... 7. Signs that a chemical change has occurred: Foaming/Fizzing Change in color Change in odor Rapid production of heat, light and/or sound Production of a new substance with different properties 8. Law of Conservation of Mass – matter is never created or destroyed in a chemical reaction. Example: If ...
... 7. Signs that a chemical change has occurred: Foaming/Fizzing Change in color Change in odor Rapid production of heat, light and/or sound Production of a new substance with different properties 8. Law of Conservation of Mass – matter is never created or destroyed in a chemical reaction. Example: If ...
The Atmosphere Atmospheric structure
... Same lapse rate > moist adiabatic lapse rate (Thunderstorm) ...
... Same lapse rate > moist adiabatic lapse rate (Thunderstorm) ...
Atomic and molecular vibrations correspond to excited
... Same lapse rate > moist adiabatic lapse rate (Thunderstorm) ...
... Same lapse rate > moist adiabatic lapse rate (Thunderstorm) ...
FHN - Chemical and Physical Changes
... Liquid evaporating to a gas Gas condensing to a liquid Liquid freezing into a solid Usually occur with a change in temperature Can also be when a substance dissolves in a liquid, but does ...
... Liquid evaporating to a gas Gas condensing to a liquid Liquid freezing into a solid Usually occur with a change in temperature Can also be when a substance dissolves in a liquid, but does ...
Chemistry I Review - BarbaraElam-Rice
... 30) An atom has 2 electrons in its valence shell. To what group does the atom belong? Will the atom for an anion or a cation? What will be the oxidation number of the ion? 31) An intermolecular force that holds ionic compounds together is called electrostatic attraction. 32) Describe the 3 intermole ...
... 30) An atom has 2 electrons in its valence shell. To what group does the atom belong? Will the atom for an anion or a cation? What will be the oxidation number of the ion? 31) An intermolecular force that holds ionic compounds together is called electrostatic attraction. 32) Describe the 3 intermole ...
Gas chromatography

Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for separating and analyzing compounds that can be vaporized without decomposition. Typical uses of GC include testing the purity of a particular substance, or separating the different components of a mixture (the relative amounts of such components can also be determined). In some situations, GC may help in identifying a compound. In preparative chromatography, GC can be used to prepare pure compounds from a mixture.In gas chromatography, the mobile phase (or ""moving phase"") is a carrier gas, usually an inert gas such as helium or an unreactive gas such as nitrogen. The stationary phase is a microscopic layer of liquid or polymer on an inert solid support, inside a piece of glass or metal tubing called a column (a homage to the fractionating column used in distillation). The instrument used to perform gas chromatography is called a gas chromatograph (or ""aerograph"", ""gas separator"").The gaseous compounds being analyzed interact with the walls of the column, which is coated with a stationary phase. This causes each compound to elute at a different time, known as the retention time of the compound. The comparison of retention times is what gives GC its analytical usefulness.Gas chromatography is in principle similar to column chromatography (as well as other forms of chromatography, such as HPLC, TLC), but has several notable differences. First, the process of separating the compounds in a mixture is carried out between a liquid stationary phase and a gas mobile phase, whereas in column chromatography the stationary phase is a solid and the mobile phase is a liquid. (Hence the full name of the procedure is ""Gas–liquid chromatography"", referring to the mobile and stationary phases, respectively.) Second, the column through which the gas phase passes is located in an oven where the temperature of the gas can be controlled, whereas column chromatography (typically) has no such temperature control. Finally, the concentration of a compound in the gas phase is solely a function of the vapor pressure of the gas.Gas chromatography is also similar to fractional distillation, since both processes separate the components of a mixture primarily based on boiling point (or vapor pressure) differences. However, fractional distillation is typically used to separate components of a mixture on a large scale, whereas GC can be used on a much smaller scale (i.e. microscale).Gas chromatography is also sometimes known as vapor-phase chromatography (VPC), or gas–liquid partition chromatography (GLPC). These alternative names, as well as their respective abbreviations, are frequently used in scientific literature. Strictly speaking, GLPC is the most correct terminology, and is thus preferred by many authors.